Sterile chromatography resin and use thereof in manufacturing processes
A resin and process technology, applied in the field of sterile chromatography resin and its use in the manufacturing process, can solve the problems of reduced flow rate, contaminated product, increased pressure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
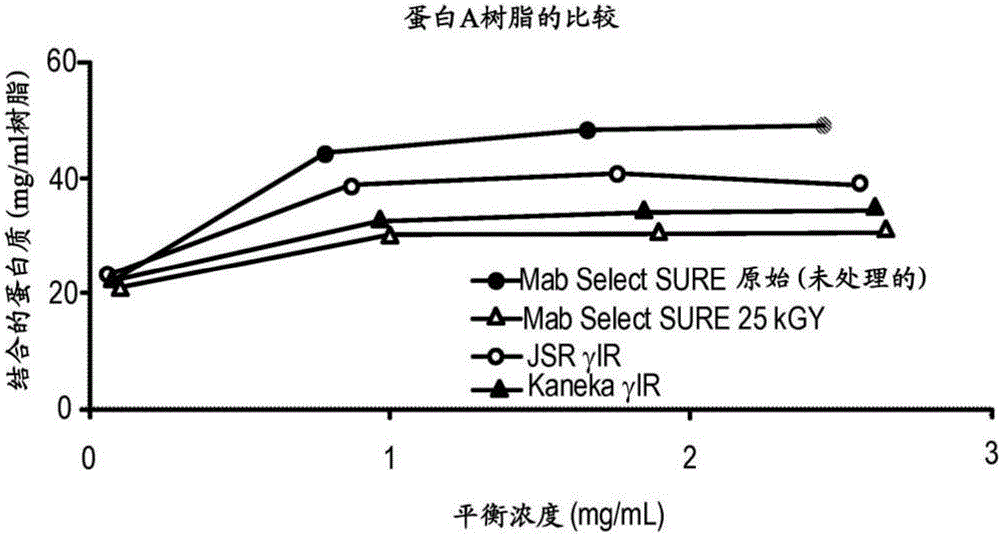
[0264] Example 1.Gamma-irradiation on the effect of static binding capacity of different chromatography resins
[0265] A set of experiments was performed to test the effect of gamma-irradiation on the static binding capacity of three different Protein A affinity chromatography resins: GE Mab Select SuRe TM (a highly cross-linked agarose resin with a particle size of 85 μm and epoxy functional groups linking protein A to the agarose), JSR LifeSciences Amsphere ProA JWT203 (a porous polymethacrylate resin with ~50 μm particle size, and epoxy functional groups linking Protein A to polymethacrylate), and Kaneka KanCap A (a highly cross-linked cellulose with a particle size of 65–85 μm in which Protein A was reductively aminated attached to cellulose). Each of the three resins was treated with 15 kGy of γ-irradiation, except Mab Select SuRe TM , which was treated with an irradiation dose of 25kGy, while the control sample GE Mab Select SuRe TM The resin was left untreated, load...
Embodiment 2
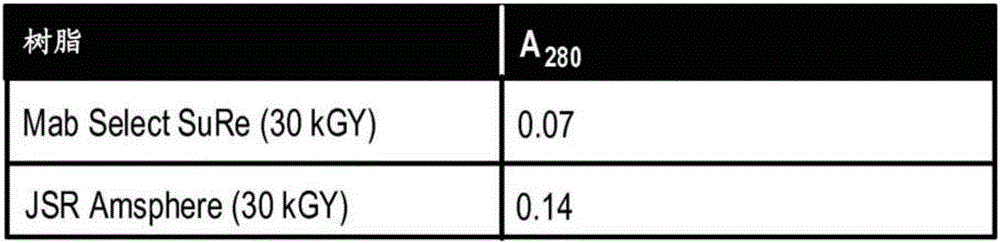
[0268] Example 2. Effect of multiple Γ-irradiation on resin binding capacity in multiple chromatography cycles
[0269] The next set of experiments was performed to test the effect of gamma-irradiation on the binding capacity of the chromatography resin in multiple chromatography cycles. JSR LifeSciences Amsphere ProA JWT203 resin was used for these experiments because this resin showed minimal loss of binding capacity in Example 1 in response to gamma-irradiation. Gamma-irradiation of each resin in this example was performed in 50 mM phosphate, pH 7.0.
[0270] A first experiment was performed to test the effect of 15 kGy γ-irradiation on the ability of JSR LifeSciences Amsphere ProA JWT203 resin to bind anti-TGFβ antibody (IgG4) in multiple chromatography cycles. Multiple chromatography cycles were also performed using untreated JSR LifeSciences Amsphere ProA JWT203 resin as a positive control. At the end of each cycle, the resin was rinsed with 0.1N NaOH. The amount of a...
Embodiment 3
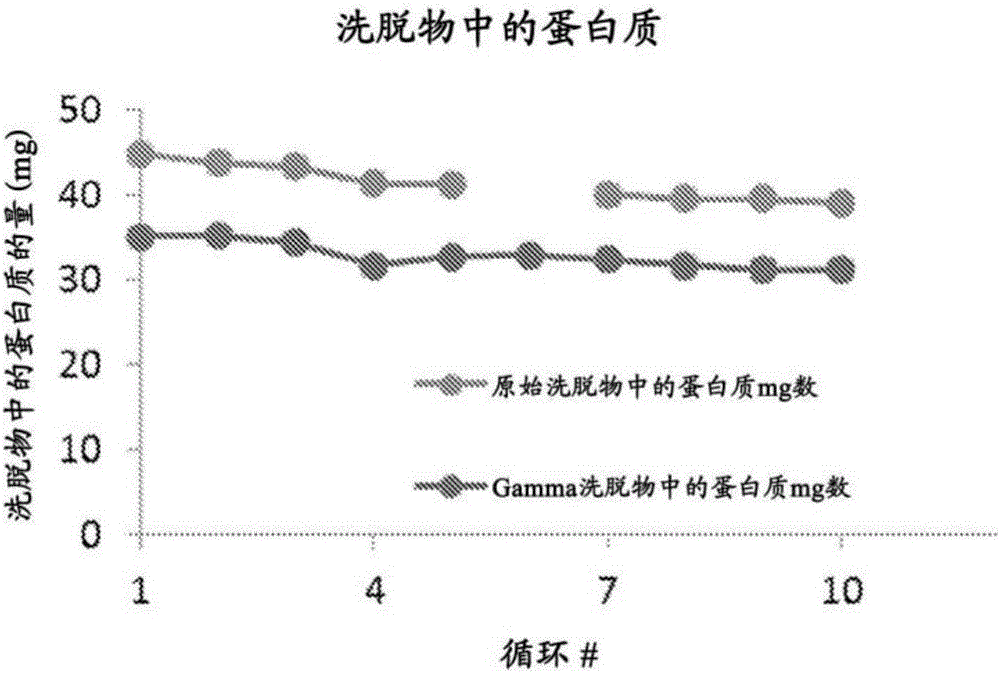
[0275] Example 3.Γ-irradiation on GE Mab Select SuRe TM The role of the binding capacity of the resin
[0276] A set of experiments was performed to determine the effect of γ-irradiation on another protein ligand chromatography resin (GE Mab Select SuRe TM Resin) binding capacity. The resin was exchanged into 50 mM sodium phosphate, pH 7.0, and then treated with 29 kGy γ-irradiation in 30-mL Nalgene bottles. The resin was then packed into a 0.66-cm diameter column (1 mL column) with a bed height of 3 cm. This column is then used to capture monoclonal IgG1 antibodies in the cell culture medium in multiple cycles of chromatography. Also used untreated GE Mab Select SuRe TM The resin was used for multiple chromatography cycles as a positive control. At the end of each cycle, the resin was rinsed with 0.1 N NaOH. The amount of IgGl in the eluate from each cycle was determined. Data show 29kGy γ-irradiation leads to GE Mab Select SuRe TM Immediate, about 25% to about 30% re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com