A method for preparing o-nitrobenzoic acid by oxidation of o-nitrotoluene with catalyst-free oxygen
A technology for o-nitrobenzoic acid and o-nitrotoluene, which is applied in the field of preparing o-nitrobenzoic acid by oxidation of o-nitrotoluene without catalyst with oxygen, can solve the problems of difficulty in catalyst synthesis, industrial application limitation, explosion danger and the like, and achieves low cost The effect of recycling, easy recycling, and easy production control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
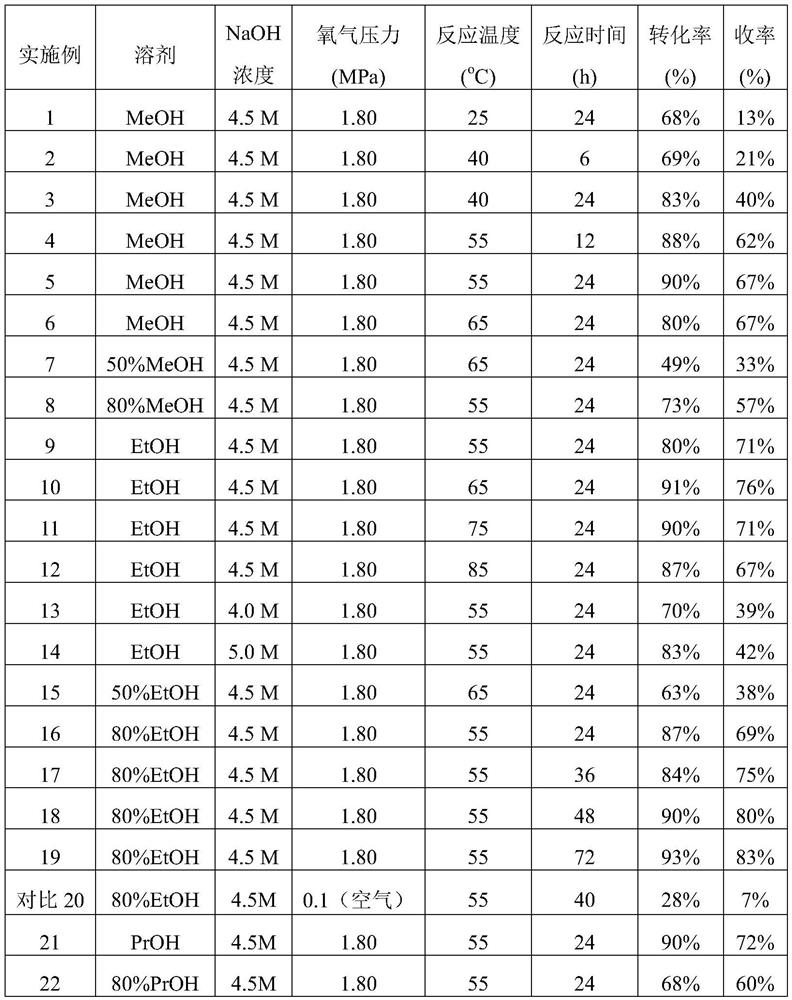
[0024]Example 1: Take o-nitrotoluene (823mg, 6mmol), sodium hydroxide (1.8g, 45mmol), put them into a 100ml autoclave, add 10ml methanol; after filling with oxygen three times, pass oxygen (pressure 1.8MPa), React in an oil bath at 25°C for 24h. After the reaction, methanol was added to dilute, the pH value of the reaction mixture was neutralized to 2-3, most of the solvent was removed under reduced pressure, ethyl acetate was added, and then dried and filtered. After chromatographic column separation, 263 mg (1.92 mmol) of o-nitrotoluene was recovered, the conversion rate of o-nitrotoluene was 68%, 130 mg (0.78 mmol) of o-nitrobenzoic acid was obtained, and the yield was 13%.
Embodiment 2
[0025]Example 2: Take o-nitrotoluene (823mg, 6mmol), sodium hydroxide (1.8g, 45mmol), put them into a 100ml autoclave, add 10ml methanol; after filling with oxygen three times, pass oxygen (pressure 1.8MPa), React in an oil bath at 40°C for 6h. After the reaction, methanol was added to dilute, the pH value of the reaction mixture was neutralized to 2-3, most of the solvent was removed under reduced pressure, ethyl acetate was added, and then dried and filtered. After chromatographic column separation, 247 mg (1.86 mmol) of o-nitrotoluene was recovered, the conversion rate of o-nitrotoluene was 69%, and 211 mg (1.26 mmol) of o-nitrobenzoic acid was obtained, and the yield was 21%.
Embodiment 3
[0026]Example 3: Take o-nitrotoluene (823mg, 6mmol), sodium hydroxide (1.8g, 45mmol), put them into a 100ml autoclave, add 10ml methanol, fill with oxygen three times, and then pass oxygen (pressure 1.8MPa). The reaction was carried out at 40°C for 24h in an oil bath. After the reaction, methanol was added to dilute, the pH value of the reaction mixture was neutralized to 2-3, most of the solvent was removed under reduced pressure, ethyl acetate was added, and then dried and filtered. After chromatographic column separation, 140 mg (1.02 mmol) of o-nitrotoluene was recovered, the conversion rate of o-nitrotoluene was 83%, and 401 mg (2.4 mmol) of o-nitrobenzoic acid was obtained, with a yield of 40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com