Method for preparing cyclic carbonate by taking NHC/ZnBr2 system as catalyst
A cyclic carbonate, catalytic preparation technology, applied in the direction of chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of production scale, single method, insufficient solution issues such as the greenhouse effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The structure of the nitrogen heterocyclic carbene (NHC) ligand involved in the present invention is as follows:
[0028]
[0029] The preparation method is as follows:
[0030]
[0031] Synthesis of N,N'-(2,6-diisopropylphenyl)-ethylenediimine 1:
[0032] In a 100mL round bottom flask, add 2,6-diisopropylaniline (8.86g, 50mmol), 40% glyoxal (3.63g, 25mmol) and 40mL absolute ethanol, and react for 10min at room temperature, It was found that a large amount of solids were precipitated. After the reaction was continued to stir for 12 hours, it was directly suction filtered and dried under vacuum to obtain 9.32 g of the target product as a yellow solid, with a yield of 99%.
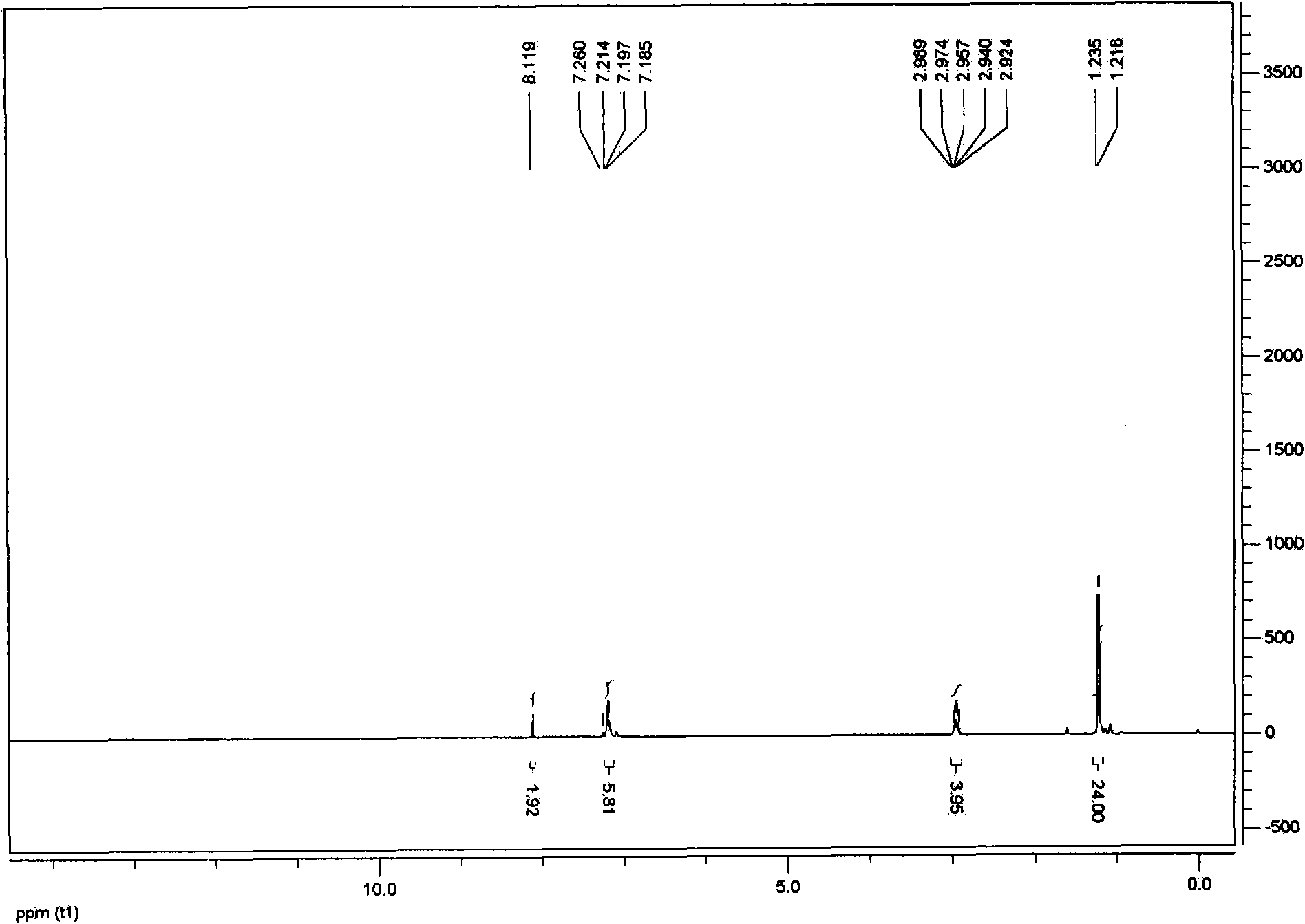
[0033] refer to figure 1 , is the hydrogen spectrum of compound 1, 1 H NMR (400MHz, CDCl 3 ): δ8.11 (s, 2H), 7.21-7.18 (m, 6H), 2.98-2.92 (m, 4H), 1.22 (d, J=6.8Hz, 24H).
[0034] Synthesis of N,N'-(2,6-diisopropylphenyl)-ethylenediamine 2:
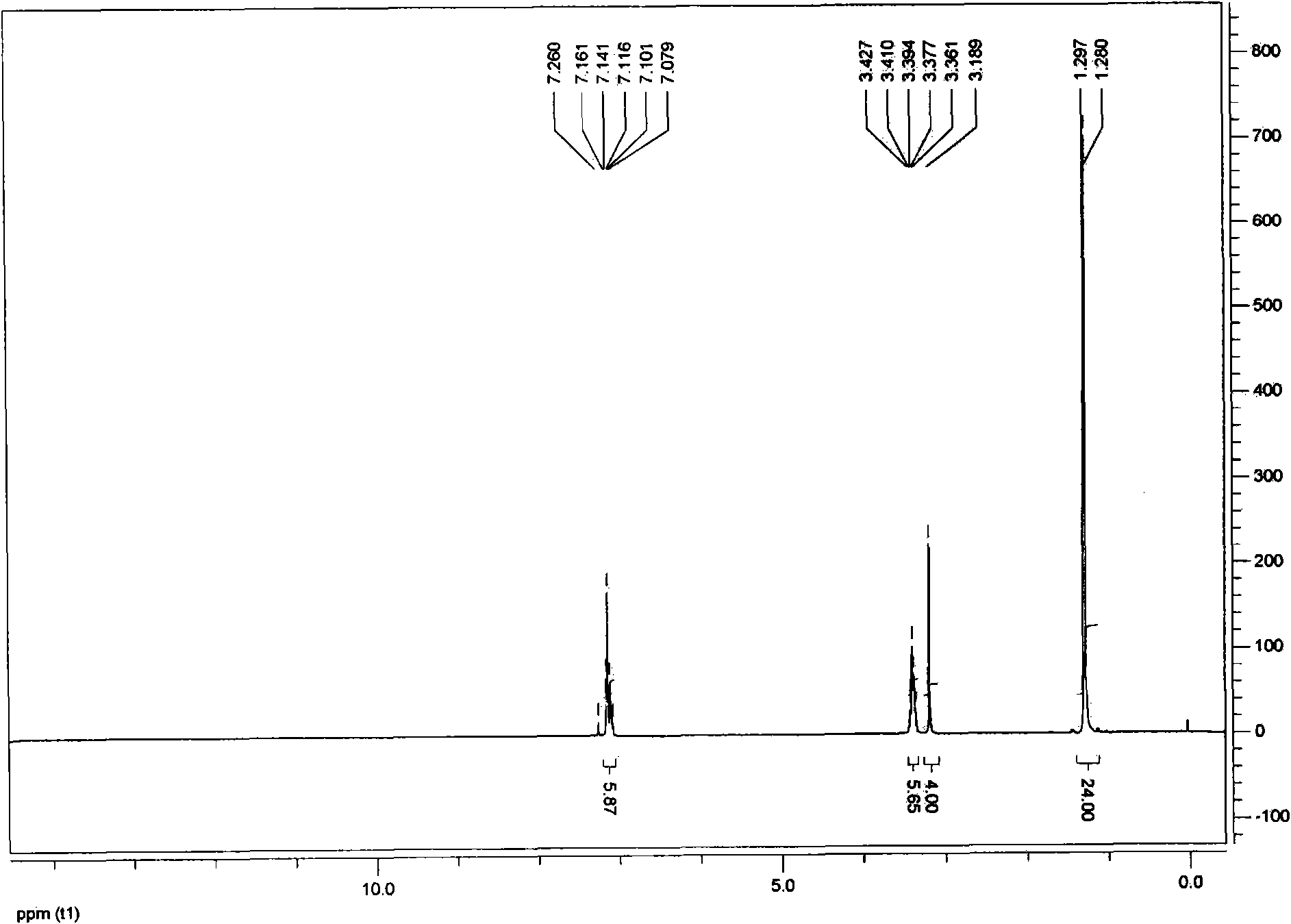
[0035] In a 250mL round bottom flask, add Compo...
Embodiment 2
[0041] NHC / ZnBr 2 The system catalyzes the cycloaddition reaction of carbon dioxide and epoxide (1,2-epoxy-butane) to prepare the corresponding cyclic carbonate. The chemical reaction formula is:
[0042]
[0043] experiment procedure
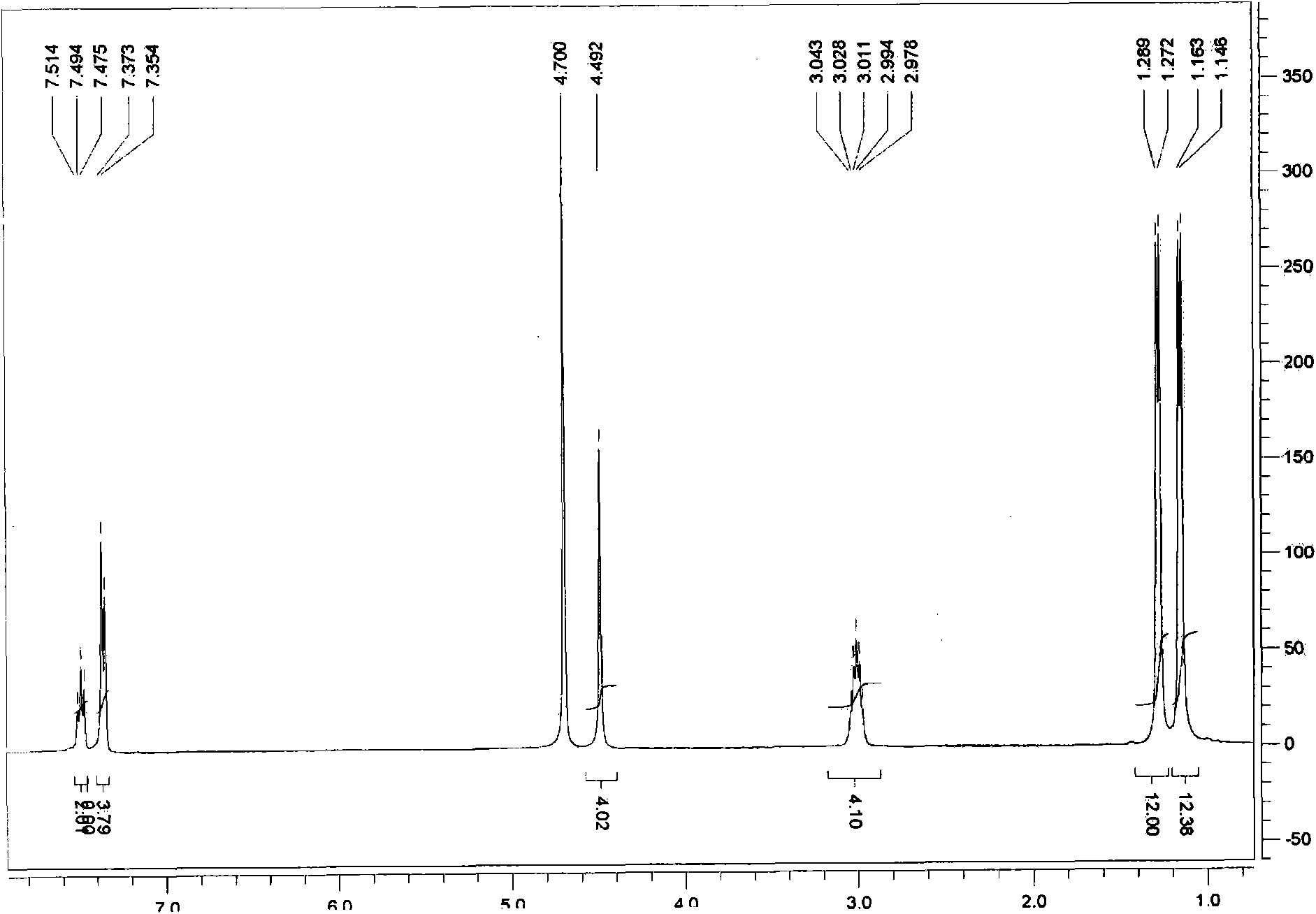
[0044] In the glove box, weigh 2mol% (the equivalent ratio of NHC to epoxide is 0.02:1) of NHC ligand (0.15g, 0.35mmol), K 2 CO 3 (0.048g, 0.35mmol), ZnBr 2 (0.079g, 0.35mmol) in a dry 50mL flask, add a stirring bar, plug a rubber stopper to seal, then place the flask on a vacuum line to evacuate, and then insert a balloon filled with high-purity carbon dioxide on the rubber stopper , Inject 10ml of anhydrous and oxygen-free DMSO into the flask with a syringe, control the pressure at 1atm, and then inject 1,2-epoxybutane (1.5mL, 17.5mmol) into the flask, and react in an oil bath at 80°C for 24h to stop the reaction. Cool to room temperature, add 200mL of deionized water, extract with dichloromethane (3 × 20mL), dry over anhydrous magnesi...
Embodiment 3
[0048] NHC / ZnBr 2 The system catalyzes the cycloaddition reaction of carbon dioxide and epoxide (1,2-epoxy n-hexane) to prepare the corresponding cyclic carbonate, the chemical reaction formula
[0049]
[0050] experiment procedure
[0051] Weigh 2mol% NHC ligand (0.15g, 0.35mmol) in the glove box, K 2 CO 3 (0.048g, 0.35mmol), ZnBr 2 (0.079g, 0.35mmol) in a dry 50mL flask, add a stirring bar, plug a rubber stopper to seal, then place the flask on a vacuum line to evacuate, and then insert a balloon filled with high-purity carbon dioxide on the rubber stopper , Inject 10ml of anhydrous and oxygen-free DMSO into the flask with a syringe, and then inject 1,2-epoxy-n-hexane (2.1mL, 17.5mmol) into the flask, react in an oil bath at 80°C for 24h to stop the reaction. Cool to room temperature, add 200mL of deionized water, extract with dichloromethane (3 × 20mL), dry over anhydrous magnesium sulfate, then obtain the crude product by rotary evaporation of the filtrate obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com