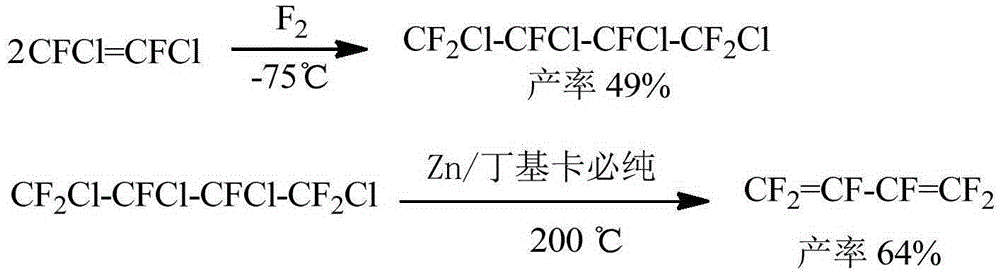
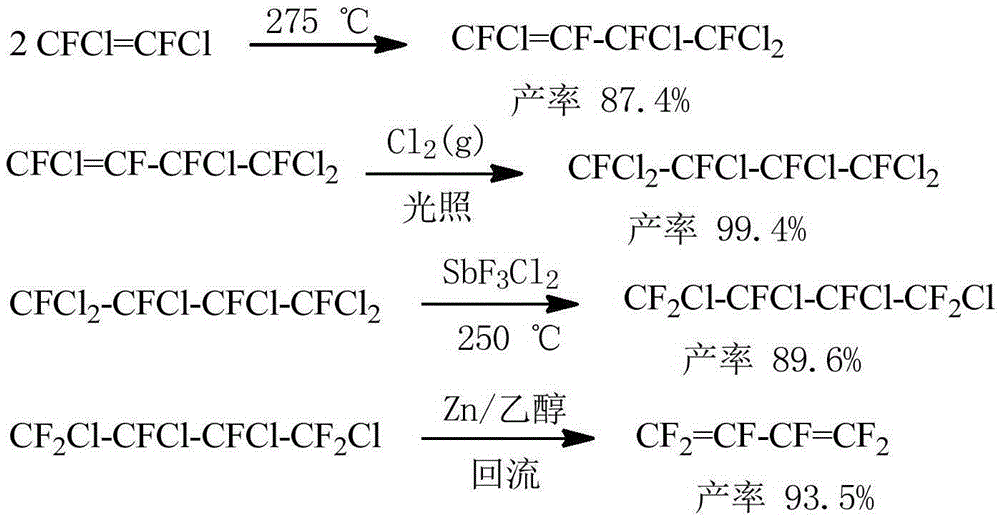
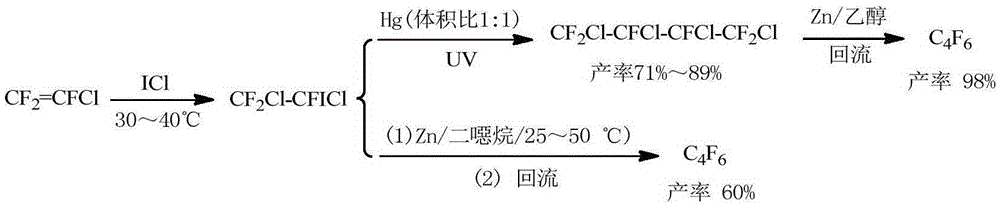
New method for preparing hexafluorobutadiene
A technology of perfluorobutadiene and a new method, applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of high chemical reaction activity, harsh reaction conditions, and many by-products, etc. The effect of obtaining, good conversion rate and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The first step: (1) in the reactor of 60 centimeters in length, the ceramic tubing of diameter 1 / 2 inch, fill 30mlFeCl 3 / C (wt, 6%) as a catalyst, (2) under the protection of nitrogen, after heating to 500 ° C in an electric furnace, HFC-134a and Br 2 Mixed and introduced into the reactor at a rate of 1:2 with a molar ratio, (3) The contact time between the reactant and the catalyst was 18 seconds. According to gas chromatography analysis, the conversion rate of HFC-134a reached 97%, CF 3 CFBr 2 The selectivity reached 62.2%; the crude product was separated and purified to obtain CF with a purity of 99.9%. 3 CFBr 2 .
[0045] Step 2: Under nitrogen protection, in a 250ml three-necked flask equipped with a thermometer and a stirrer, add 20g of activated zinc powder and 40ml of dried DMF (moisture content below 50ppm), heat to 60°C, and stir Slowly add CF under the condition 3 CFBr 2 27g, adjust the rate of addition, keep the reaction temperature below 90°C during ...
Embodiment 2
[0048] The first step: (1) in the reactor of 60 centimeters in length, the ceramic tubing of diameter 1 / 2 inch, fill 30mlCuCl 2 / C (wt, 6%) as a catalyst, (2) under the protection of nitrogen, after heating to 550 ° C in an electric furnace, HFC-134a and Br 2 Mixed and introduced into the reactor at a rate of 1:3 in molar ratio, (3) The reaction contact time between the reactant and the catalyst was 18 seconds. According to gas chromatography analysis, the conversion rate of HFC-134a reached 99%, CF 3 CFBr 2 The selectivity reached 70.5%; the crude product was separated and purified to obtain CF with a purity of 99.9%. 3 CFBr 2 .
[0049] Step 2: Under nitrogen protection, in a 250ml three-necked flask equipped with a thermometer and a stirrer, add 20g of activated zinc powder and 40ml of dried NMP (moisture content below 50ppm) respectively, heat to 60°C, and stir Slowly add CF under the condition 3 CFBr 2 27g, adjust the rate of addition, keep the reaction temperature ...
Embodiment 3
[0052] The first step: (1) in length 60 centimeters, in the reactor of internal helical stainless steel tubing of diameter 1 / 2 inch, fill 30mlFeBr 3 / C (wt, 6%) as a catalyst, (2) under the protection of nitrogen, after heating to 550 ° C in an electric furnace, HFC-134a and Br 2 Mixed and introduced into the reactor at a rate of 1:3 in molar ratio, (3) The reaction contact time between the reactant and the catalyst was 15 seconds. According to gas chromatography analysis, the conversion rate of HFC-134a reached 99.5%, CF 3 CFBr 2 The selectivity reached 75.5%; the crude product was separated and purified to obtain CF with a purity of 99.9%. 3 CFBr 2 .
[0053] The second step: under nitrogen protection, in a 250ml three-necked flask equipped with a thermometer and a stirrer, add 25g of activated zinc powder and 45ml of dried acetonitrile (water content below 50ppm) respectively, heat to 60°C, and Slowly add CF dropwise under stirring condition 3 CFBr 2 25g, adjust the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com