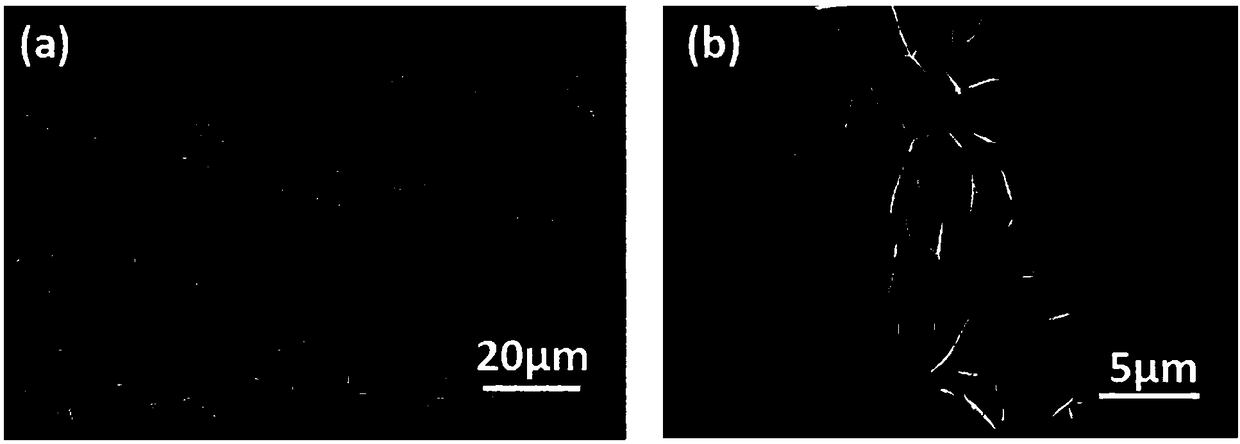
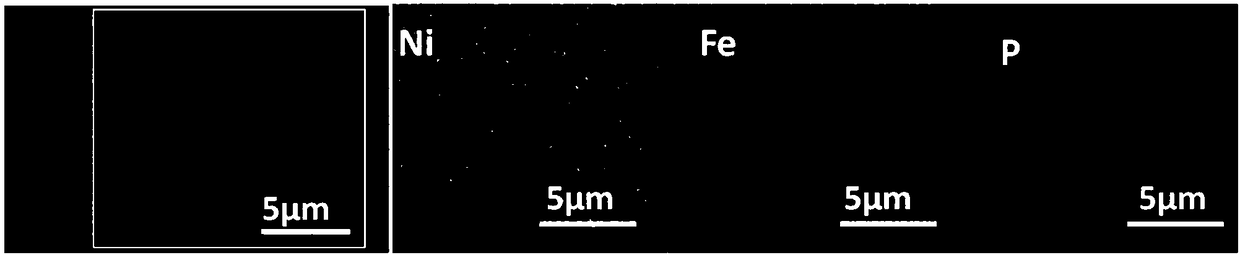
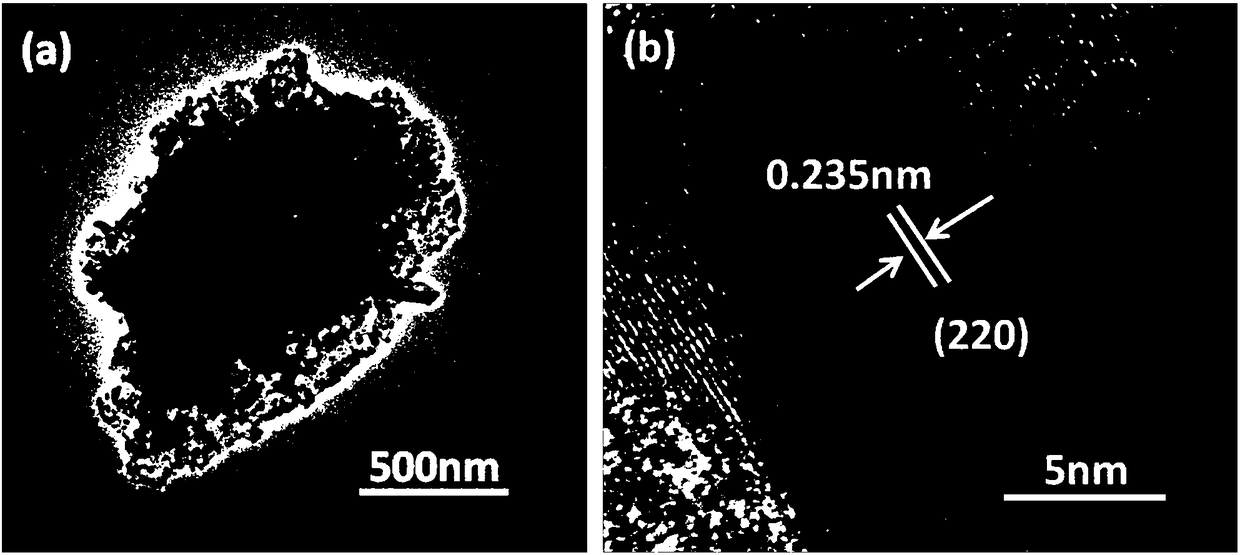
Ternary nickeliron phosphidenanosheet, preparation method thereof and application of electrolytic water
A technology of iron nanometer and nickel phosphide, applied in chemical instruments and methods, phosphide, nanotechnology, etc., can solve the problems that hinder large-scale application, limited active sites, low conductivity of LDHs, etc., and achieve low cost and crystallization Good performance and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Ni x Fe (1-x) P 2 Preparation of nanosheets
[0030] (1) The carbon cloth (CF) was ultrasonically cleaned with deionized water, ethanol, and acetone, and dried at 60 °C for 7 h. Immerse the cleaned CF into 40mL containing 0.654g nickel nitrate hexahydrate (Ni(NO 3 ) 2 ·6H 2 O), 0.303g ferric nitrate nonahydrate (Fe(NO 3 ) 3 9H 2 O) and 0.9g urea (CO(NH 2 ) 2 ), placed in a 50mL reactor and raised to 100°C for 6 hours, cooled to room temperature for washing after the reaction, and dried at 60°C to obtain Ni x Fe (1-x) (OH) 2 Nanosheets.
[0031] (2) P powder and Ni grown on carbon cloth x Fe (1-x) (OH) 2The nanosheets are respectively put into the small diameter tube (inner tube) and the large diameter tube (outer tube) of the quartz sleeve, and the large diameter tube and the small diameter tube are set so that the front and rear ends of the quartz sleeve are respectively placed in dual temperature zones In the upstream and downstream central temperatur...
Embodiment 2
[0044] Ni x Fe (1-x) P 2 Preparation of nanosheets
[0045] (1) The carbon cloth (CF) was ultrasonically cleaned with deionized water, ethanol, and acetone, and dried at 60 °C. Immerse the washed CF into 40mL containing 0.741g nickel nitrate hexahydrate Ni(NO 3 ) 2 ·6H 2 O, 0.182g iron nitrate nonahydrate Fe(NO 3 ) 3 9H 2 O and 0.9g urea (CO(NH 2 ) 2 ), placed in a 50mL reactor and raised to 100°C for 6 hours, cooled to room temperature for washing after the reaction, and dried at 60°C to obtain Ni x Fe (1-x) (OH) 2 Nanosheets.
[0046] (2) P powder and the prepared Ni x Fe (1-x) (OH) 2 The nanosheets were placed at both ends of the casing respectively, and placed in the upstream and downstream central temperature zones of the dual-temperature zone tubular reaction furnace. After the quartz tube was repeatedly cleaned with argon (Ar), the temperature of the upstream furnace was 400°C, and the temperature of the downstream furnace was raised to 400°C. As high a...
Embodiment 3
[0050] Ni x Fe (1-x) P 2 Preparation of nanosheets
[0051] (1) The carbon cloth (CF) was ultrasonically cleaned with deionized water, ethanol, and acetone, and dried at 60 °C. Immerse the washed CF into 40mL containing 0.567g nickel nitrate hexahydrate Ni(NO 3 ) 2 ·6H 2 O, 0.424g iron nitrate nonahydrate Fe (NO 3 ) 3 9H 2O and 0.9g urea (CO(NH 2 ) 2 ), placed in a 50mL reactor and raised to 100°C for 6 hours, cooled to room temperature for washing after the reaction, and dried at 60°C to obtain Ni x Fe (1-x) (OH) 2 Nanosheets.
[0052] (2) P powder and the prepared Ni x Fe (1-x) (OH) 2 The nanosheets were placed at both ends of the casing respectively, and placed in the upstream and downstream central temperature zones of the dual-temperature zone tubular reaction furnace. After the quartz tube was repeatedly cleaned with argon (Ar), the temperature of the upstream furnace was 400°C, and the temperature of the downstream furnace was raised to 400°C. As high a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com