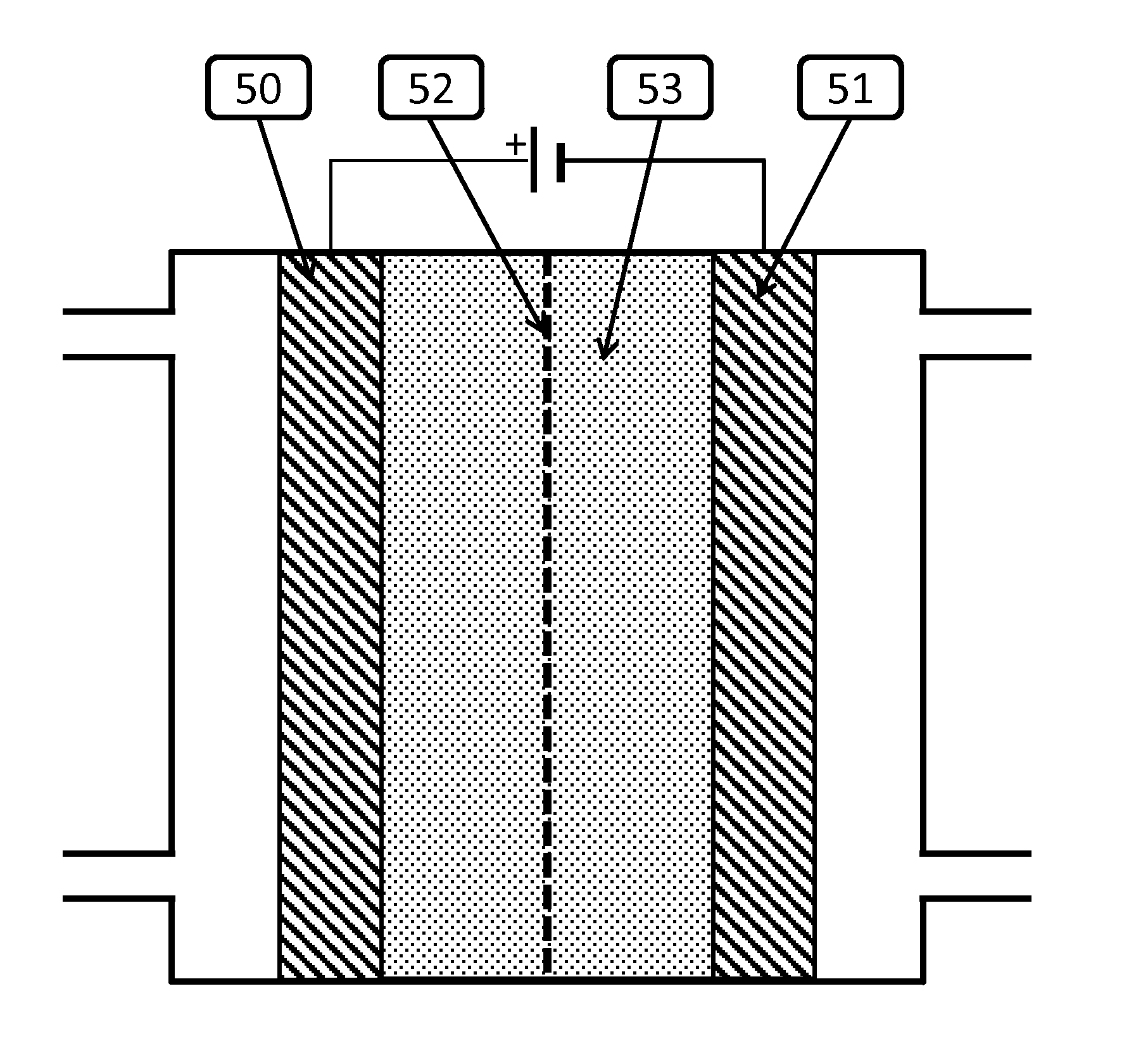
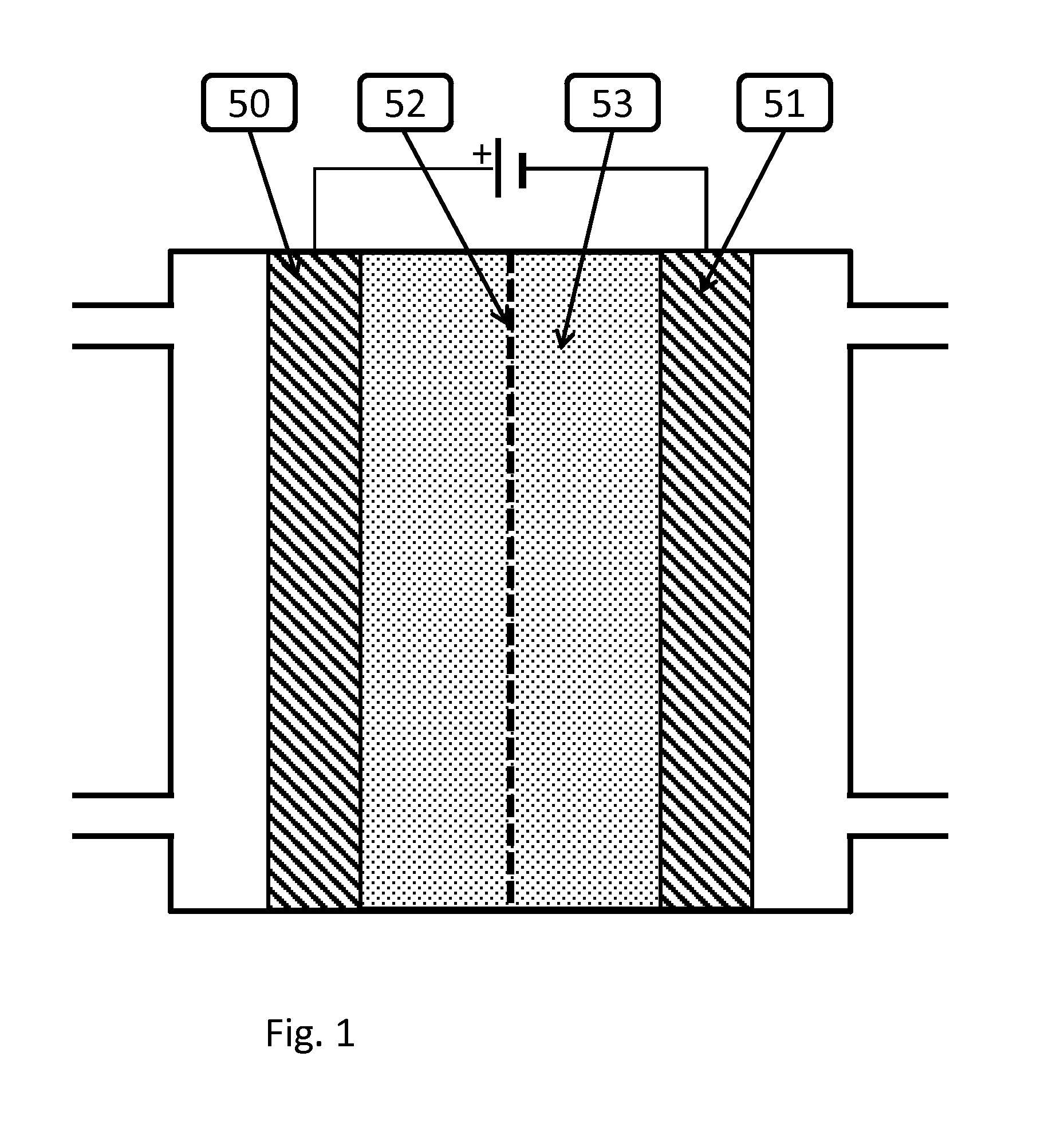
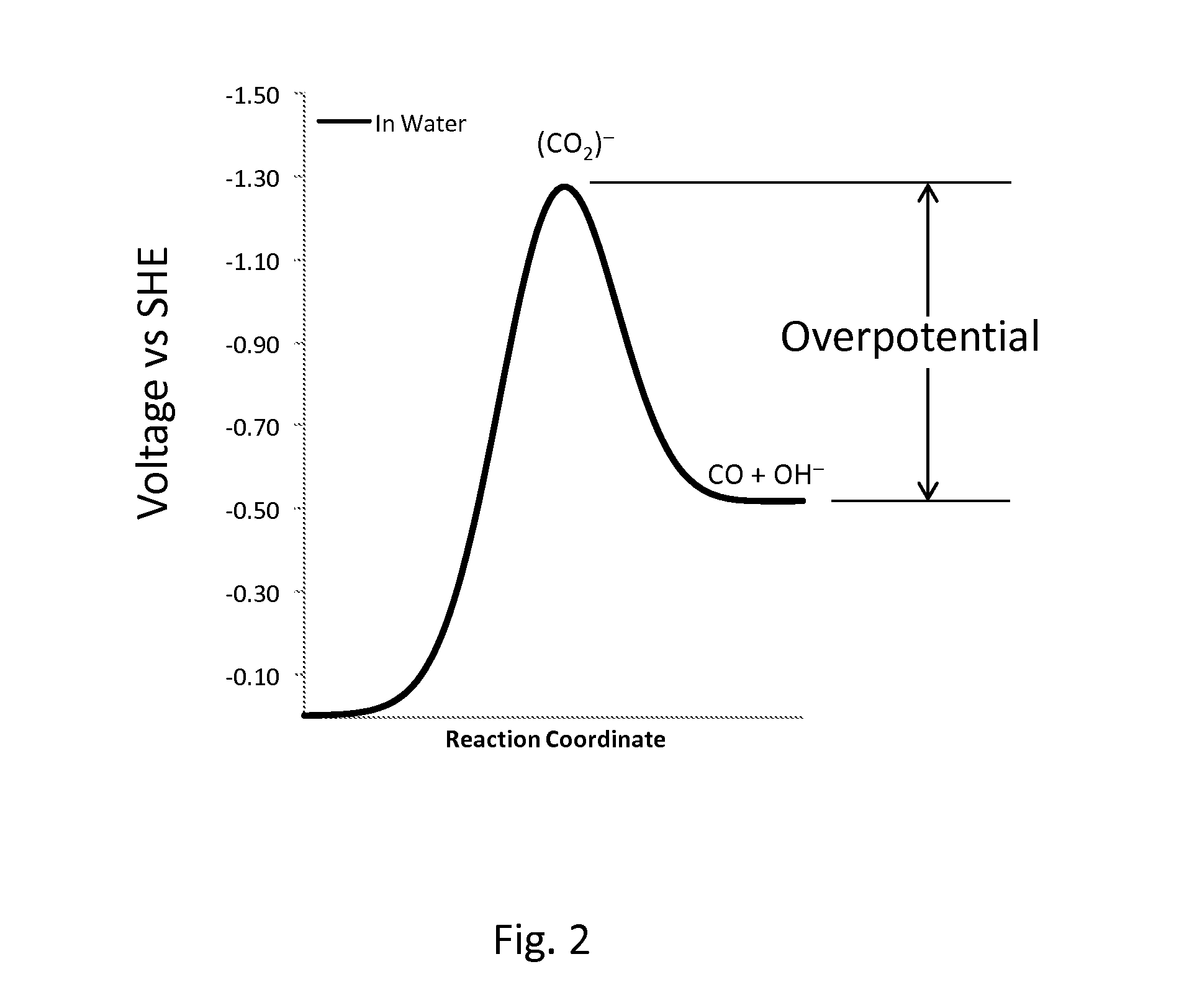
Novel catalyst mixtures
a catalyst and mixture technology, applied in the field of catalysts, can solve the problems of preventing efficient conversion of carbon dioxide into, lack of catalysts, and high overpotential costs, and achieve the effects of low electron conversion efficiency, high overpotentials, and low rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific example 1
Using a Active Element, Helper Catalyst Mixture including of Platinum and 1-ethyl-3-Methylimidazoilum Tetrafluoroborate (EMIM-BF4) to Lowering the Overpotential for Electrochemical Conversion of CO2 and Raising the Selectivity (Current Efficiency) of the Reaction
[0078]The experiments used the glass three electrode cell shown in FIG. 7. The cell consisted of a Three neck flask (101), to hold the anode (108), and the cathode (109). A silver / 0.01 molar silver ion reference electrode (103) in acetnonitrile was connected to the cell through a Luggin Cappillary (102). The reference electrode (103) was fitted with a vycor frit to prevent any of the reference electrode solution from contaminating the ionic liquid in the capillary. The reference electrode was calibrated against the Fc / Fc+ redox couple. A conversion factor of +535 was used convert our potential axis to reference the Standard Hydrogen Electrode (SHE). A 25×25 mm Platinum gauze (size 52) (113) was connected to the anode while a...
specific example 2
The Effect of Dilution on the Electrochemical Conversion of CO2
[0090]This example shows that water additions speed the formation of CO. The experiment used the Cell and procedures in Example 1, with the following exception: a solution containing 98.55% EMIM-BF4 and 0.45% water was substituted for the 99.9999% EMIM-BF4 used in Example 1, the potential was held for 10 or 30 minutes at −0.6V with respect to RHE, and then the potential was ramped positively at 50 mV / sec. FIG. 10 shows the result. Notice the peak at between 1.2 and 1.5 eV. This is the peak associated with CO formation and is much larger than in example 1. Thus the addition of water has accelerated the formation of CO presumably by acting as a reactant.
specific example 3
Using a Active Element, Helper Catalyst Mixture that include Palladium and choline Iodide to Lowering the Overpotential for Electrochemical Conversion of CO2 in Water
[0091]The next example is to demonstrate that the invention can be practiced using Palladium as an active element and Choline Iodide as a Helper Catalyst.
[0092]The experiment used the cell and procedures in Example 1, with the following exceptions: ii) A 10.3% by weight of a Helper Catalyst, choline iodide in water solution was substituted for the 1-ethyl-3-methylimidazolium tetrafluoroborate and ii) a 0.25 cm2 Pd foil purchased from Alfa Aesar was substituted for the gold plug and platinum black on the cathode, and a silver / silver chloride reference was used.
[0093]FIG. 11 shows a CV taken under these conditions. There is a large negative peak near zero volts with respect with SHE associated with iodine transformations and a negative going peak starting at about 0.8 V associated with conversion of CO2. By comparison the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com