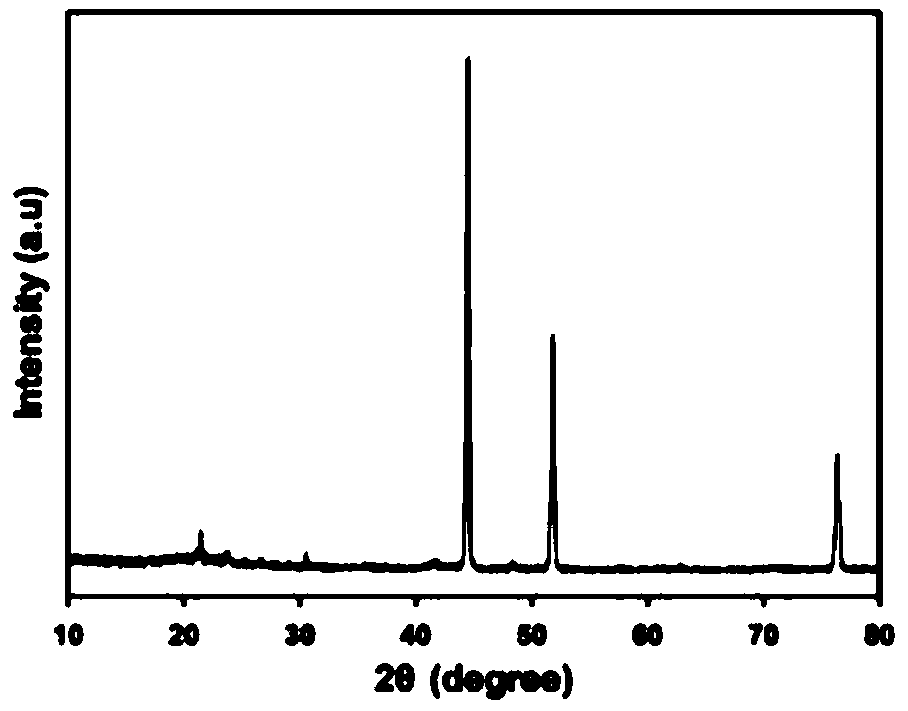
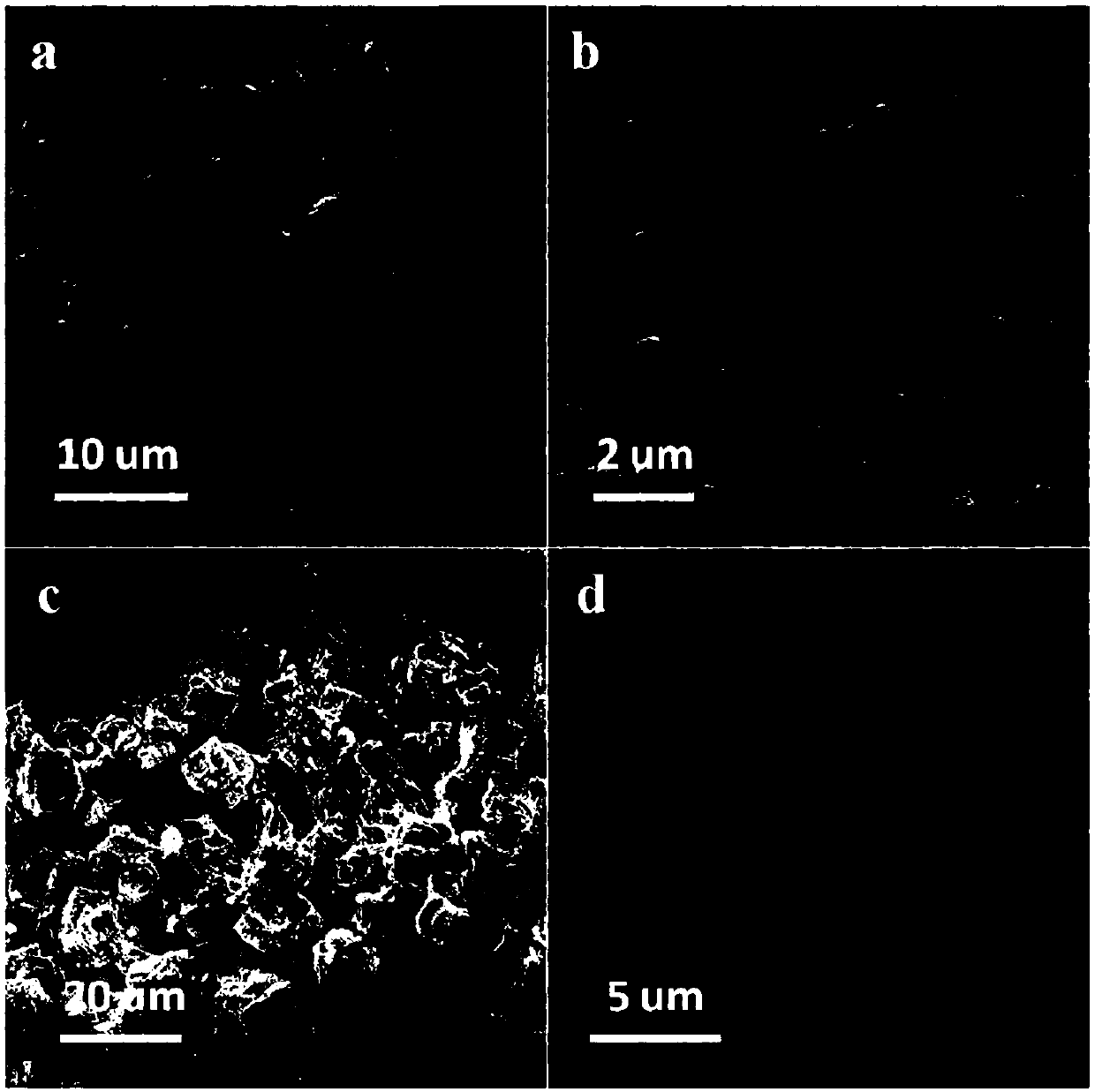
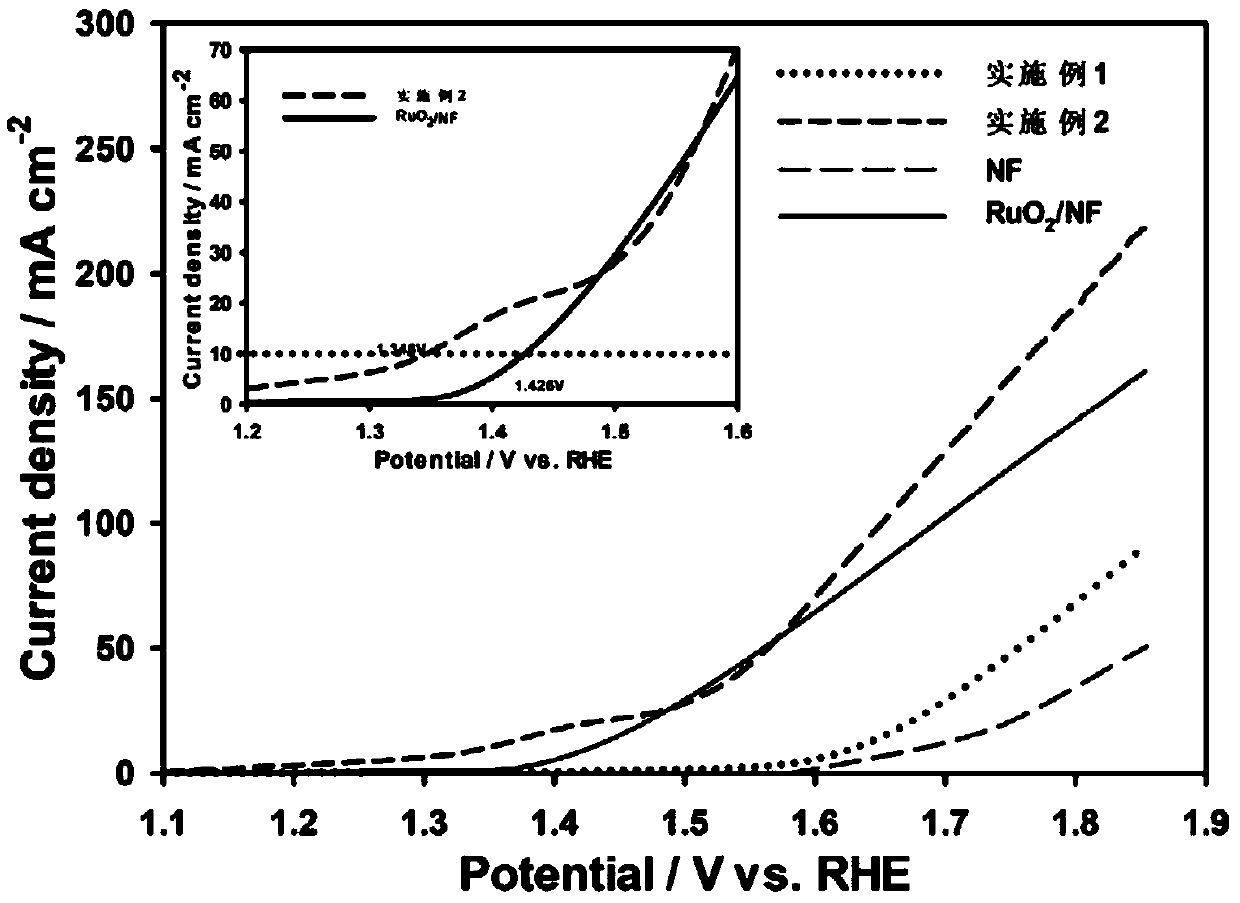
Transition metal nitride/carbon electrocatalyst and preparation method and application of transition metal nitride/carbon electrocatalyst
A transition metal, electrocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, electrodes, etc., can solve the problems of large overpotential, single hydrogen evolution or oxygen evolution performance of catalyst materials, etc., to achieve excellent electrical performance, excellent The effect of electrocatalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Forming the shell directly on the surface of the substrate is as follows:
[0092]Dissolve 0.2mmol copper nitrate trihydrate in 40mL N,N-dimethylformamide (DMF) to form reaction solution A, dissolve 1mmol 2-aminoterephthalic acid in 15mL DMF to form reaction solution B, and dissolve the reaction solution B was added to reaction solution A, stirred by magnetic force for 15 minutes, then transferred to a polytetrafluoroethylene lining, then added a piece of cleaned foam nickel, sealed and placed in a stainless steel autoclave and reacted at 120 ° C for 24 hours, naturally Cool to room temperature (25°C). The product was washed three times with absolute ethanol and water, and then dried at 60°C to obtain the composite precursor of copper metal organic framework and nickel foam; the above composite precursor of copper metal organic framework and nickel foam was placed in a tube furnace, and the Under an ammonia atmosphere, set the heating rate at 5°C / min, hold temperature ...
Embodiment 2
[0094] Add 3mmol of urea to 80ml of 1mM cobalt chloride solution, stir until completely dissolved into a transparent solution, add a piece of clear and clean nickel foam, package it in a reaction kettle, set the reaction temperature to 120°C, and the reaction time to 10h. The final product was washed several times with water and alcohol, filtered and dried at 60°C to obtain a composite precursor of cobalt hydroxide and nickel foam (precursor 1); 0.5 mmol of copper nitrate trihydrate was dissolved in 15 mL of DMF to form a reaction solution A , 1mmol 2-aminoterephthalic acid was dissolved in 15mL DMF to form reaction solution B, reaction solution B was added to reaction solution A, and after magnetic stirring for 15 minutes, it was transferred to a polytetrafluoroethylene liner, followed by the above preparation to obtain hydrogen The composite layer of cobalt oxide and nickel foam (precursor 1) was sealed and placed in a stainless steel autoclave and reacted at 120°C for 24 hou...
Embodiment 3
[0096] Compared with Example 2, the main difference is that the calcination temperature is changed to 500°C, as follows:
[0097] Add 3mmol of urea to 80ml of 1mM cobalt chloride solution, stir until completely dissolved into a transparent solution, add a piece of clear and clean nickel foam, package it in a reaction kettle, set the reaction temperature to 120°C, and the reaction time to 10h. The final product was washed several times with water and alcohol, and dried at 60°C after filtration to obtain a composite precursor of cobalt hydroxide and nickel foam; 0.5 mmol of copper nitrate trihydrate was dissolved in 15 mL of DMF to form a reaction solution A, and 1 mmol of 2- Aminoterephthalic acid was dissolved in 15mL DMF to form reaction solution B, and reaction solution B was added to reaction solution A. After magnetic stirring for 15 minutes, it was transferred to a polytetrafluoroethylene liner, and then cobalt hydroxide and nickel foam were prepared as above The composit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com