Preparation method of N-doped porous carbon coated nano-particles of Co-Ir core-shell structure and application of N-doped porous carbon coated nano-particles of Co-Ir core-shell structure to catalytic water splitting
A technology of nitrogen-doped porous carbon and core-shell structure, which is applied in the electrolysis process, electrolysis components, transportation and packaging, etc., can solve the problems that have not yet been published in the literature or patent reports, and achieve low price, high catalytic performance, and low cost. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
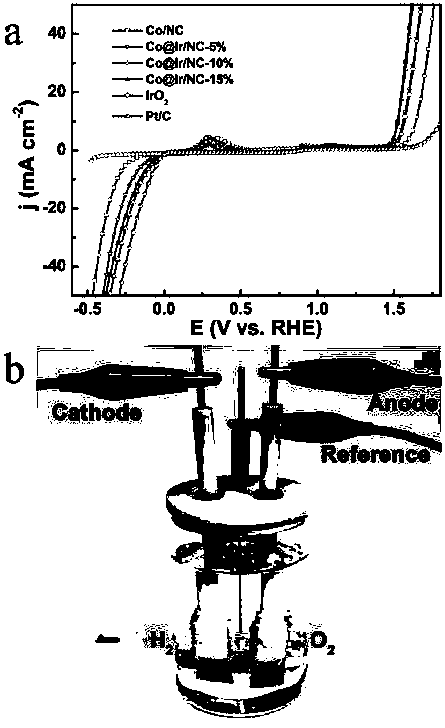
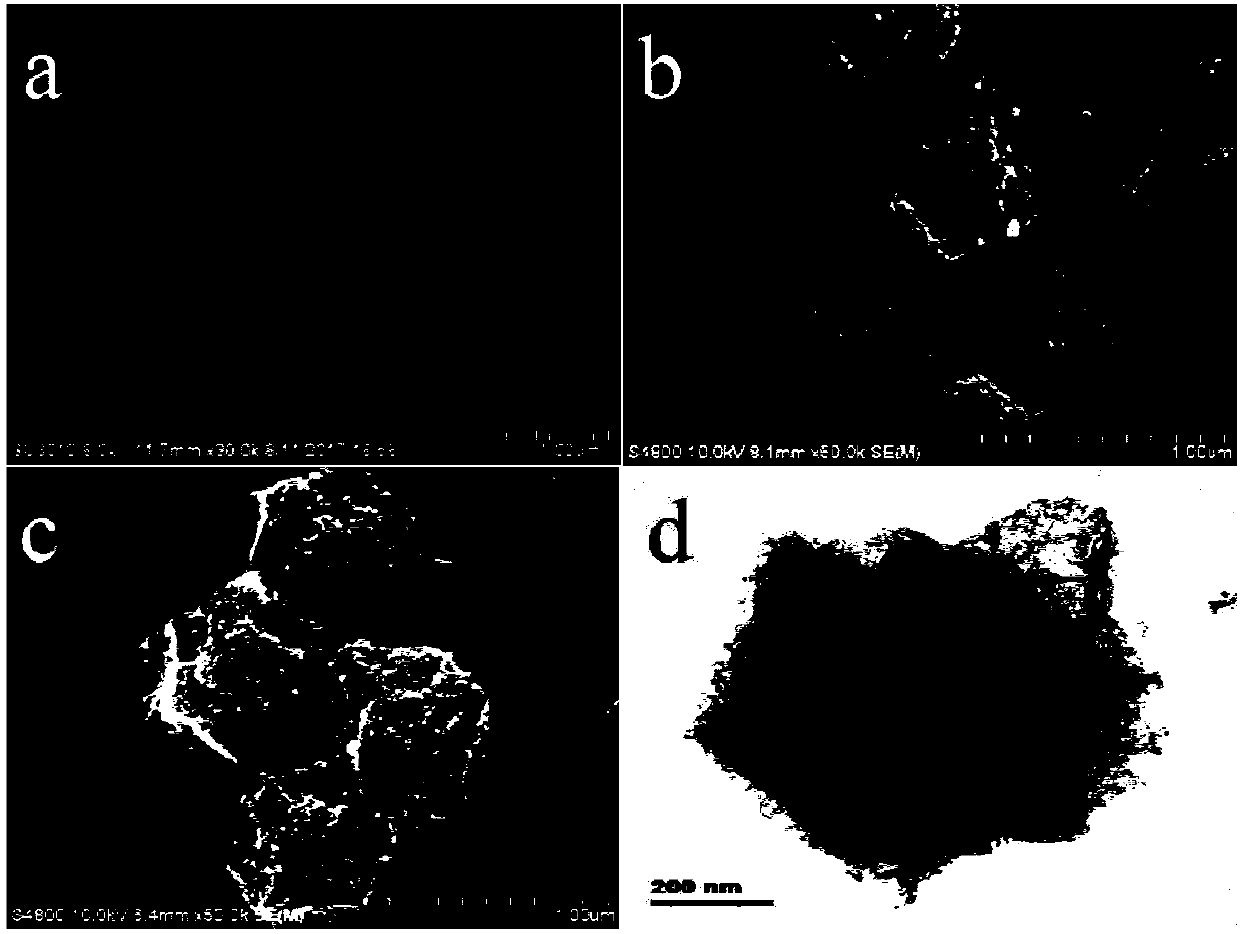
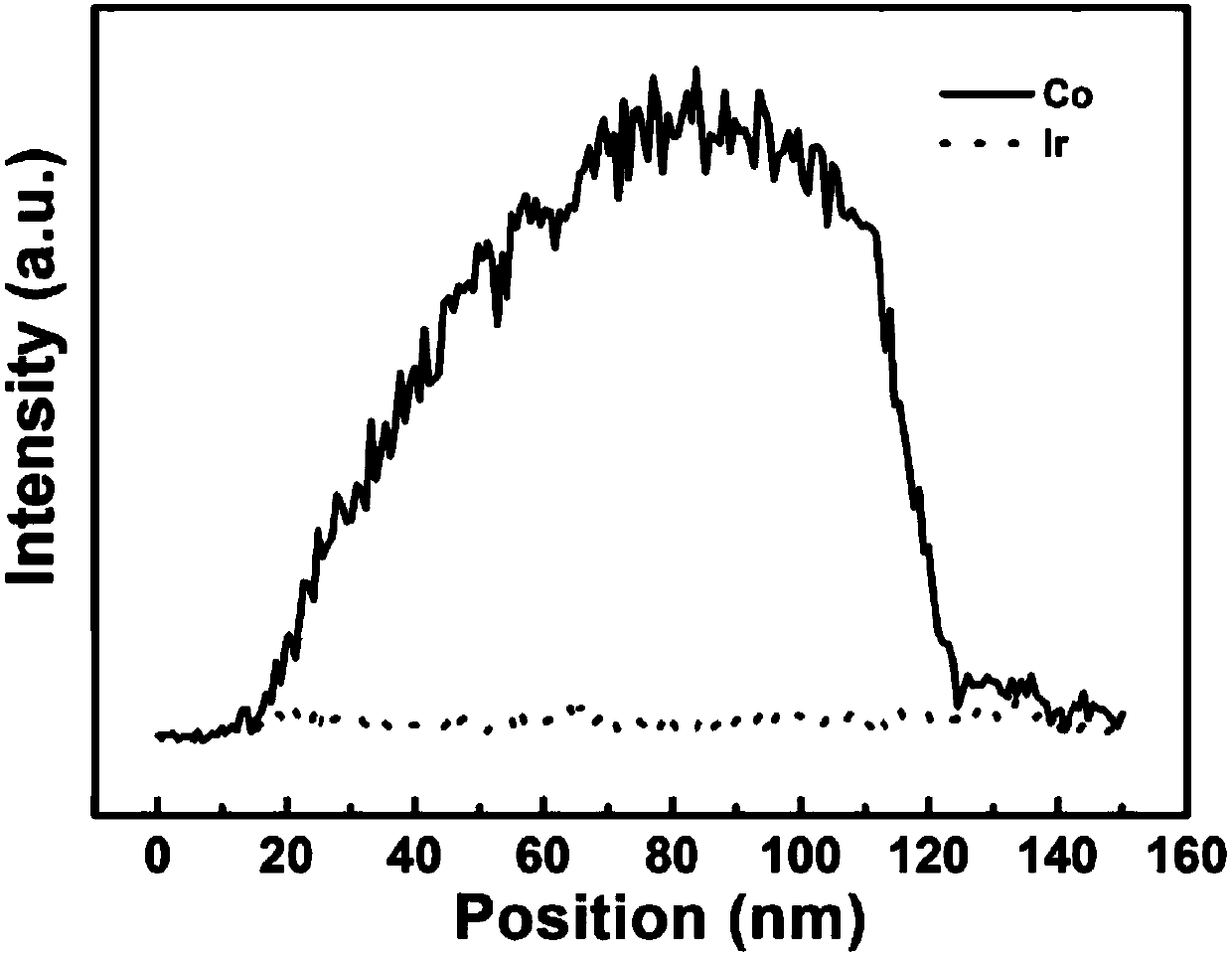
[0036] (1) According to the molar ratio Co 2+ : MeIM=1:4 ratio, at room temperature, 6.98g Co(NO 3 ) 2 ·6H 2 Dissolve O in 240mL of methanol to make solution A, then dissolve 7.88g of 2-methylimidazole in 80mL of methanol to make solution B; mix and stir the two solutions of A and B for 10min, let stand for 24h, then centrifuge, and wash with methanol Several times; the resulting dark blue precipitate was dried in a vacuum oven at 50° C. for 12 hours to obtain ZIF-67 nanocrystals. figure 1 The ZIF-67 nanocrystal shown in a is a polyhedral geometry with a size of 840-1100 nm.
[0037] (2) Place the ZIF-67 nanocrystal obtained above in a tube furnace, heat it to 900° C. under an Ar gas atmosphere and keep it warm for 3 hours, and cool to room temperature after the reaction. The obtained black powder is nitrogen-doped porous carbon immobilized Cobalt nanoparticles (Co-NC). figure 1 The Co-NC particles shown in b are polyhedral geometry with rough surface.
[0038] (3) Take ...
Embodiment 2
[0053] Same as in Example 1, except that the amount of iridium chloride was reduced to 4.38 mg to obtain Co@Ir / NC-5%. Resulting material properties:
[0054] The specific surface area is 135.61m 2 g -1 ;
[0055] At a current density of 10mA cm -2 , the required overpotential for the oxygen evolution reaction is 322mV;
[0056] Oxygen evolution reaction Tafel slope is 78.3mV dec -1 ;
[0057] At a current density of 10mA cm -2 , the overpotential required for the hydrogen evolution reaction is -198mV;
[0058] The Tafel slope of the hydrogen evolution reaction is 142.9mV dec -1 .
Embodiment 3
[0060] Same as in Example 1, except that the amount of iridium chloride was increased to 15.98 mg to obtain Co@Ir / NC-15%. Resulting material properties:
[0061] The specific surface area is 135.61m 2 g -1 ;
[0062] At a current density of 10mA cm -2 , the required overpotential for the oxygen evolution reaction is 302mV;
[0063] Oxygen evolution reaction Tafel slope is 76.3mV dec -1 ;
[0064] At a current density of 10mA cm -2 , the overpotential required for the hydrogen evolution reaction is -147mV;
[0065] The Tafel slope of the hydrogen evolution reaction is 133.2mV dec -1 .
[0066] The electrochemical tests of Examples 2 and 3 are the same as Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com