Preparation method and application of cobalt sulfur compound
A cobalt-sulfur compound and reaction technology, applied in cobalt compounds, chemical instruments and methods, cobalt sulfide, etc., can solve problems such as difficult application of energy storage systems, easy agglomeration of materials, and poor cycle performance of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Material preparation:
[0066] Add 1g of cobalt chloride and 2g of urea to a mixed solution of 80mL of deionized water and glycerol at a mass ratio of 1:2, stir for a period of time until the solution is clear, transfer the mixed solution to a polytetrafluoroethylene lining, The inner lining is placed in a high-pressure reaction kettle, and the temperature is naturally raised to 120°C in a blast oven, and reacted for 12 hours. After it is lowered to room temperature, it is taken out for multiple filtration and washing. The washed product is dried in a vacuum oven at 40-60°C for 12-24 hours.
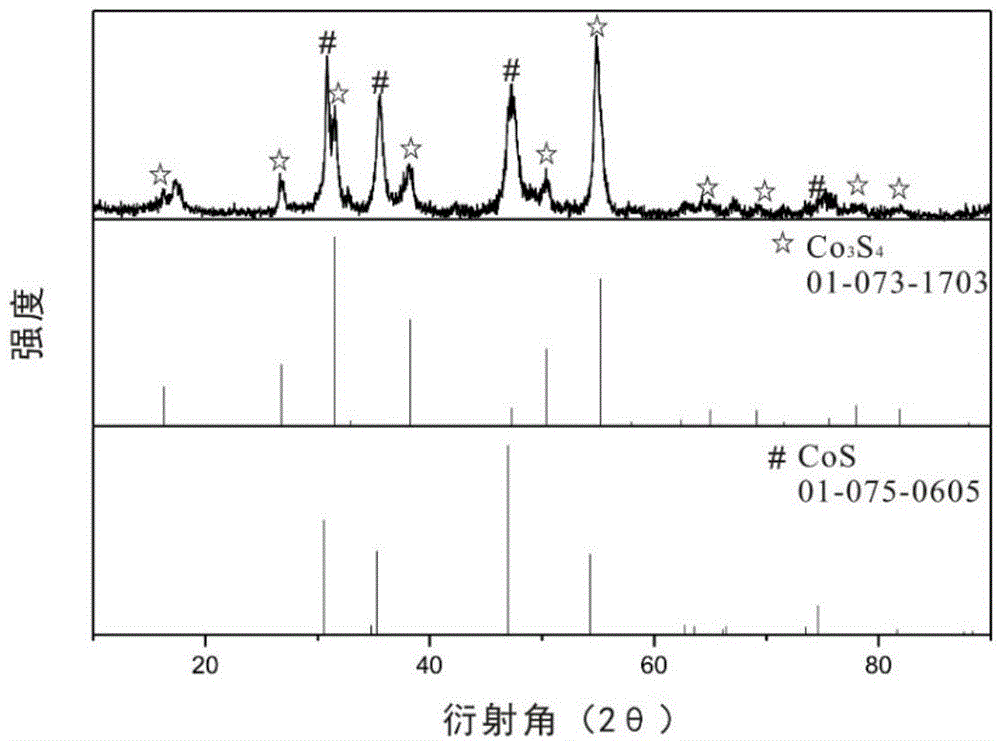
[0067] Put 1 g of the sample obtained in the above process in a muffle furnace for calcination at 500°C for 3 hours, then place it under a reducing atmosphere, add 1.5 g of sulfur source, and heat at 350°C for 6 hours to obtain the cobalt-sulfur compound with a specific shape .
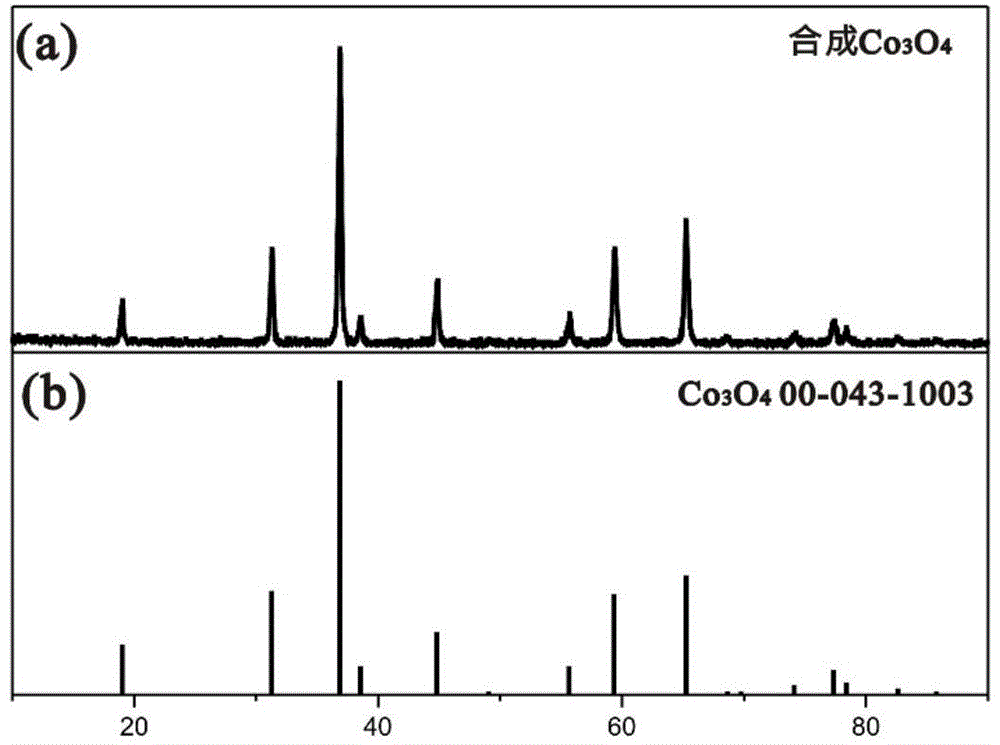
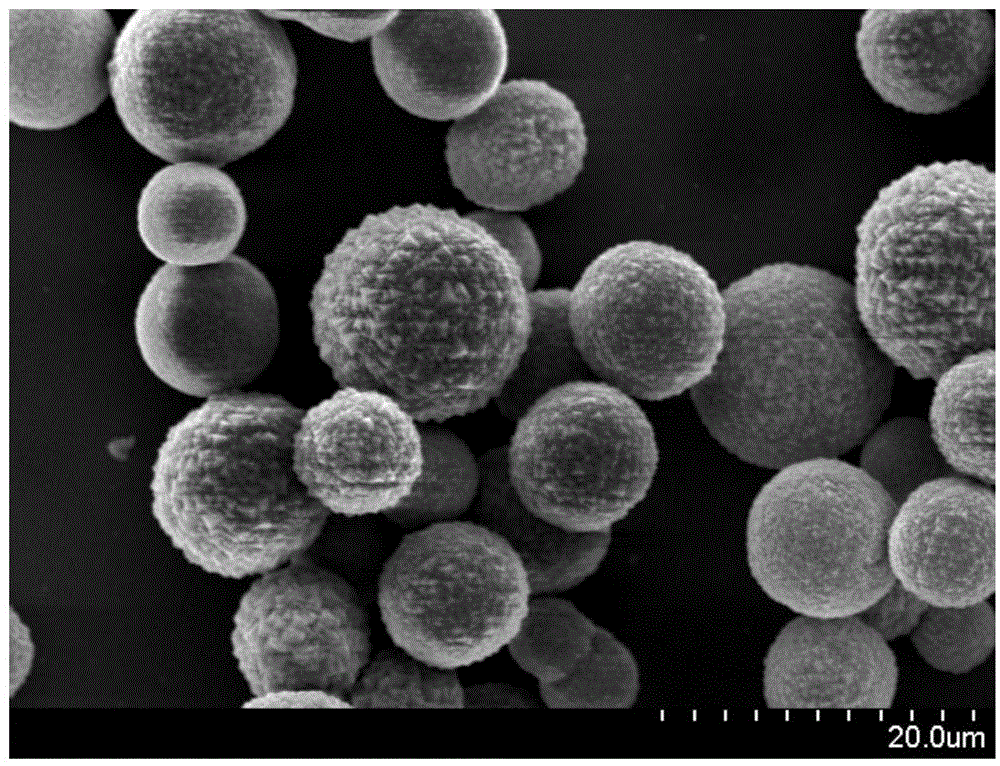
[0068] Material Characterization:
[0069] The morphology of the material was analyzed by a scanning...
Embodiment 2
[0074] Material preparation:
[0075] Add 1g of cobalt chloride and 8g of urea to a mixed solution of 80mL of deionized water and glycerol according to the mass ratio of 1:8, stir for a period of time until the solution is clear, and transfer the mixed solution to a polytetrafluoroethylene lining, The inner lining is placed in a high-pressure reaction kettle, and the temperature is naturally raised to 120°C in a blast oven, and reacted for 12 hours. After it is lowered to room temperature, it is taken out for multiple filtration and washing. The washed product is dried in a vacuum oven at 40-60°C for 12-24 hours. Put 1g of the sample obtained in the above process into a muffle furnace for calcination at 400°C for 3 hours, then put it under a reducing atmosphere, add 1.5g of sulfur source, and heat at 350°C for 6h to obtain the cobalt-sulfur with specific morphology compound.
[0076] Material Characterization:
[0077] The morphology of the material was analyzed by a scanni...
Embodiment 3
[0082] Material preparation:
[0083] Dissolve a certain amount of cobalt salt, urea, and triethanolamine in a certain amount of deionized water to prepare solutions with concentrations of 25, 30, and 20 mg / mL, stir for a period of time until the solution is clear, and transfer the liquid to polytetrafluoroethylene Lining, the lining is placed in a high-pressure reaction kettle, and the temperature is naturally raised to 160°C in a blast oven, and reacted for 12 hours. After it is lowered to room temperature, it is taken out for multiple filtration and washing. The washed product is dried in a vacuum oven at 40-60°C for 12-24 hours.
[0084] Place the sample obtained in the above process in a muffle furnace for 6 hours at 600°C to calcinate to obtain cobalt oxide, then place 1 g of cobalt oxide in a reducing atmosphere, add 2 g of thiourea, and react at 350°C for 6 hours to obtain the Specific morphology of cobalt sulfur compounds.
[0085] Material Characterization:
[008...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com