Electrocatalyst with cobalt-based multi-stage nano-composite structure for oxygen production by electrolysis of water and preparation method of electrocatalyst
A nanocomposite, electrocatalyst technology, applied in chemical instruments and methods, cobalt compounds, nanotechnology, etc., can solve problems such as speeding up, and achieve the effects of simple operation, reduced cost, and increased active area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
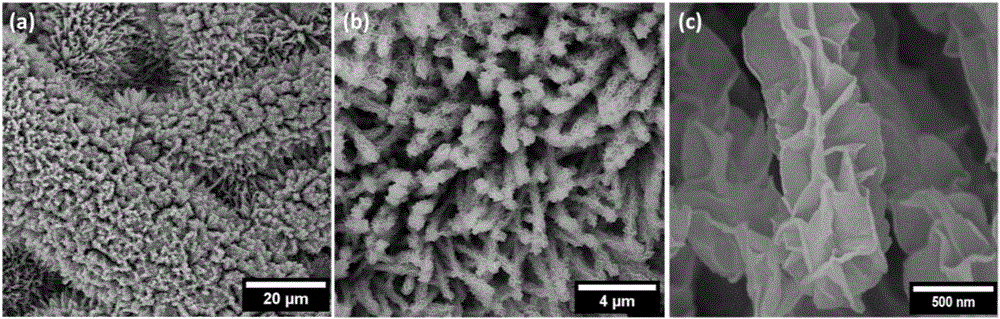
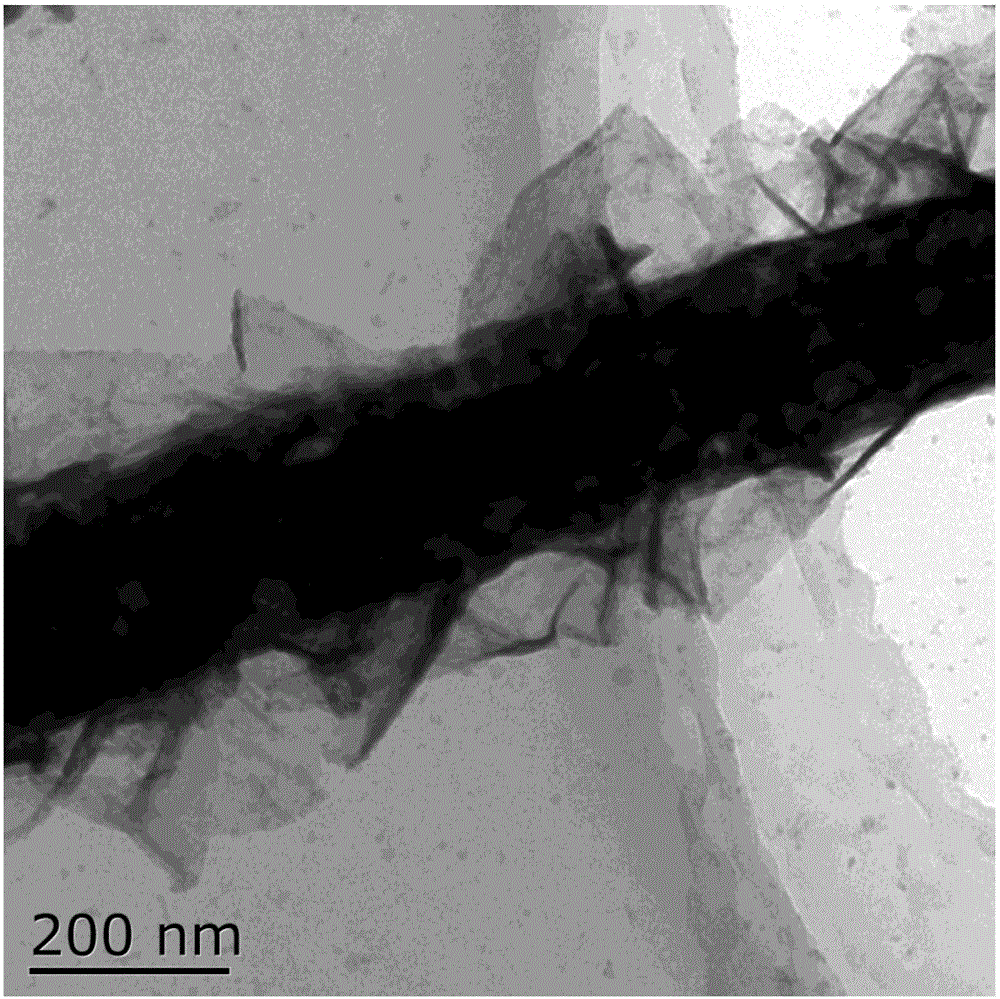
[0030] Solvothermal reaction: dissolve 0.87g cobalt nitrate hexahydrate, 0.90g urea, and 0.22g ammonium fluoride in 80mL water, put them into a 100mL hydrothermal kettle, and put washed 2×6cm 2 carbon fiber paper, and then put the hydrothermal kettle into a constant temperature oven at 120°C, keep it warm for 7 hours, cool it naturally, take it out, wash it, and dry it to get the composite structure of basic cobalt carbonate nanowires loaded on carbon fiber paper.
[0031] Low-temperature vulcanization reaction: use 2g of sulfur powder as the sulfur source, raise the temperature at 8°C / min to 450°C under nitrogen (flow rate 100sccm), keep it warm for 60min and then cool naturally. The carbon fiber paper supported cobalt sulfide nanowire composite structure was obtained.
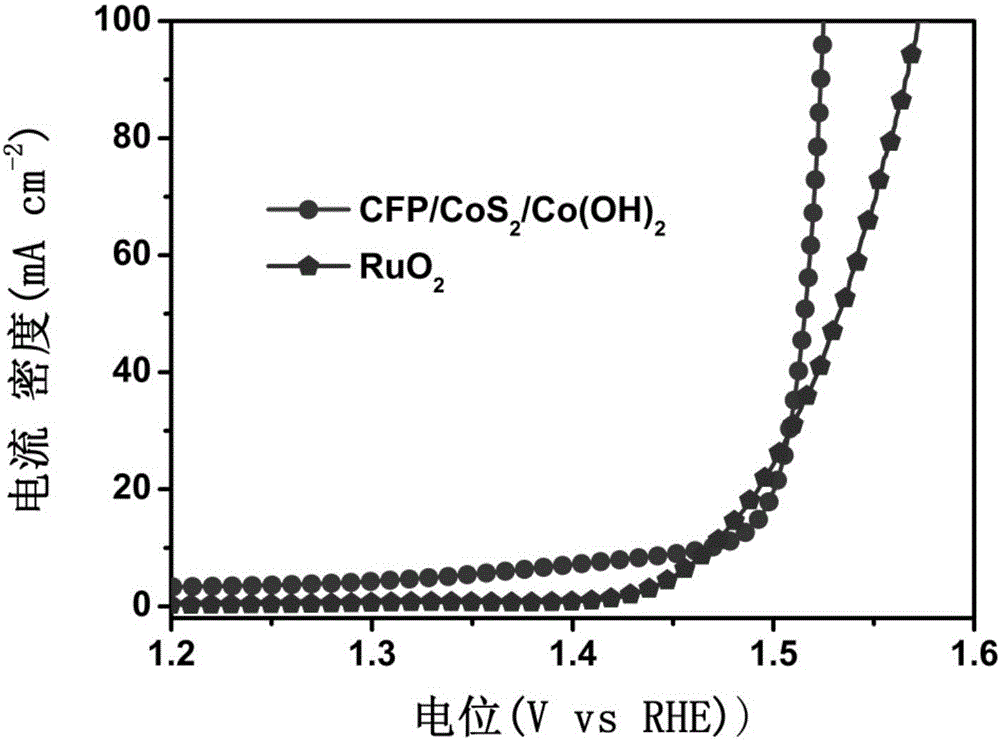
[0032] Electrochemical deposition by cyclic voltammetry: using a three-electrode system, carbon fiber paper supported cobalt sulfide nanowire composite structure as the working electrode, platinum as the auxi...
Embodiment 2
[0034] The difference from Example 1 is:
[0035] Solvothermal reaction: dissolve 0.87g cobalt nitrate hexahydrate, 0.90g urea, and 0.22g ammonium fluoride in 80mL water, put them into a 100mL hydrothermal kettle, and put washed 2×6cm 2 carbon fiber paper, and then put the hydrothermal kettle into a constant temperature oven at 120°C, keep it warm for 10 hours, cool it naturally, take it out, wash it, and dry it to get a carbon fiber paper-loaded basic cobalt carbonate nanowire composite structure.
[0036] Low-temperature vulcanization reaction: use 2g of sulfur powder as the sulfur source, raise the temperature at 8°C / min to 450°C under nitrogen (flow rate 100sccm), keep it warm for 60min and then cool naturally. The carbon fiber paper supported cobalt sulfide nanowire composite structure was obtained.
[0037] Electrochemical deposition by cyclic voltammetry: using a three-electrode system, carbon fiber paper supported cobalt sulfide nanowire composite structure as the wor...
Embodiment 3
[0039] The difference from Example 1 is:
[0040] Solvothermal reaction: dissolve 0.87g cobalt nitrate hexahydrate, 0.90g urea, and 0.22g ammonium fluoride in 80mL water, put them into a 100mL hydrothermal kettle, and put washed 2×6cm 2 carbon fiber paper, and then put the hydrothermal kettle into a constant temperature oven at 120°C, keep it warm for 12 hours, cool it naturally, take it out, wash it, and dry it to get the carbon fiber paper-loaded basic cobalt carbonate nanowire composite structure.
[0041] Low-temperature vulcanization reaction: use 2g of sulfur powder as the sulfur source, raise the temperature at 8°C / min to 450°C under nitrogen (flow rate 100sccm), keep it warm for 60min and then cool naturally. The carbon fiber paper supported cobalt sulfide nanowire composite structure was obtained.
[0042]Electrochemical deposition by cyclic voltammetry: using a three-electrode system, carbon fiber paper supported cobalt sulfide nanowire composite structure rice wire...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com