Controllable preparation method of Co-Mo-S (cobalt-molybdenum-sulfur) ternary metal sulfide
A ternary metal, co-mo-s technology, applied in chemical instruments and methods, cobalt sulfide, cobalt compounds, etc., can solve the problems of difficult control of the growth of composite components, difficult controllable preparation, etc. The effect of strong operability and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
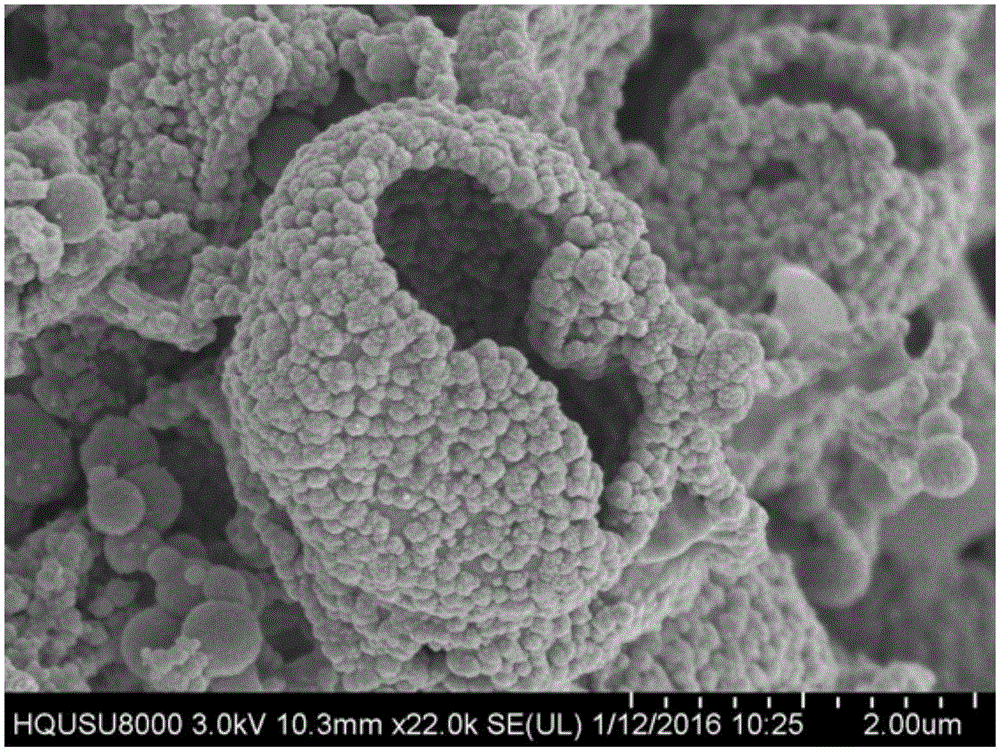
Image
Examples
Embodiment 1
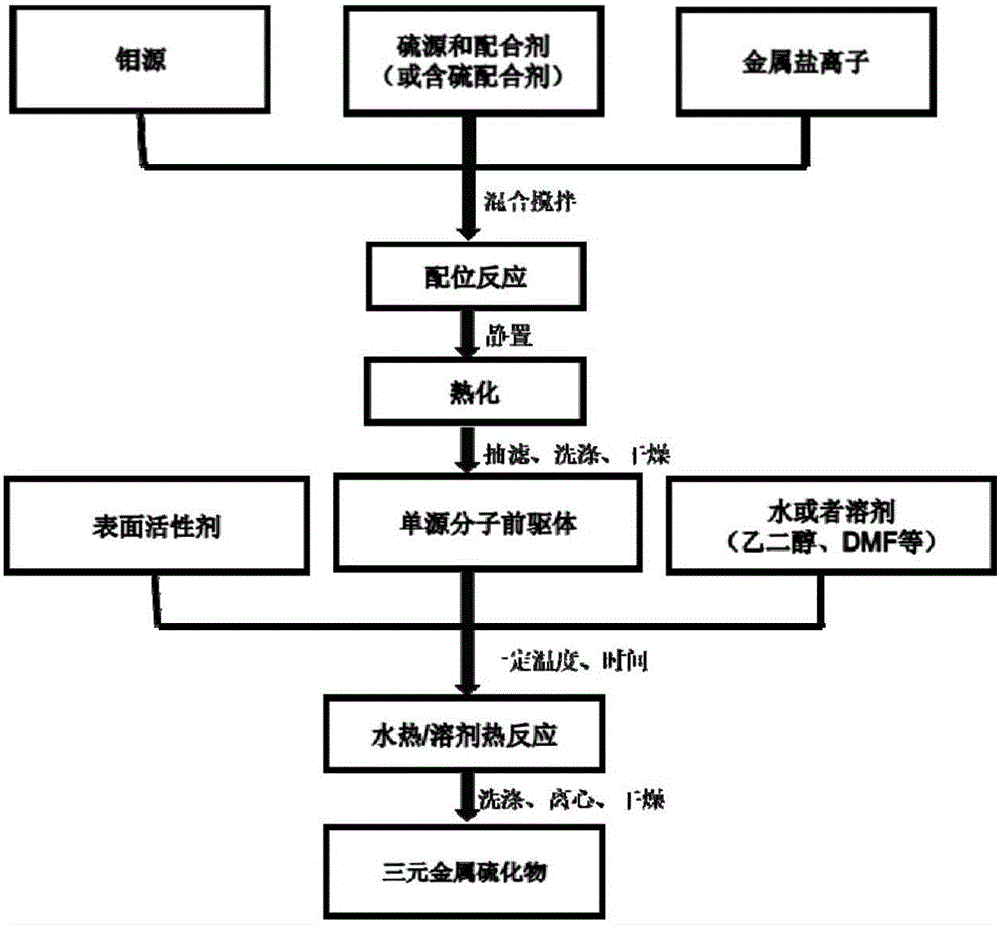
[0028] (1) Single-source molecular precursor MoO 2 (DDTC) 3 Preparation of Co
[0029] Weigh 0.4g ammonium paramolybdate [(NH 4 ) 6 Mo 7 o 24 4H 2 O] and 0.6g cobalt nitrate hexahydrate [Co(NO 3 ) 2 ·6H 2 O] was dissolved in 100ml of distilled water, stirred magnetically for 0.5 hour, and the solution formed was designated as solution A. Weigh 1.0 g of sodium diethyldithiocarbamate (DDTC) and dissolve it in 100 ml of distilled water, and magnetically stir for 0.5 hour, and the formed solution is recorded as B solution. The solution A was slowly added dropwise to the solution B, and stirred magnetically for 4.5 hours, and the formed solution was recorded as solution C. Stop stirring solution C, let it stand still for 12 hours, filter it with suction, wash it with distilled water and absolute ethanol three times, and dry it in a blast drying oven at 60°C for 12 hours to obtain a dry powder sample, which is a single-source molecular precursor.
[0030] (2) Preparation ...
Embodiment 2
[0033] (1) Single-source molecular precursor MoO 2 (DDTC) 3 Preparation of Co
[0034] Weigh 0.4g ammonium paramolybdate [(NH 4 ) 6 Mo 7 o 24 4H 2 O] and 0.6g cobalt nitrate hexahydrate [Co(NO 3 ) 2 ·6H 2 O] was dissolved in 100ml of distilled water, stirred magnetically for 0.5 hour, and the solution formed was designated as solution A. Weigh 1.0 g of sodium diethyldithiocarbamate (DDTC) and dissolve it in 100 ml of distilled water, and magnetically stir for 0.5 hour, and the formed solution is recorded as B solution. The solution A was slowly added dropwise to the solution B, and stirred magnetically for 4.5 hours, and the formed solution was recorded as solution C. Stop stirring solution C, let it stand still for 12 hours, filter it with suction, wash it with distilled water and absolute ethanol three times, and dry it in a blast drying oven at 60°C for 12 hours to obtain a dry powder sample, which is a single-source molecular precursor.
[0035] (2) Preparation ...
Embodiment 3
[0038] (1) Single-source molecular precursor MoO 2 (DDTC) 3 Preparation of Co
[0039]Weigh 0.4g ammonium paramolybdate [(NH 4 ) 6 Mo 7 o 24 4H 2 O] and 0.6g cobalt nitrate hexahydrate [Co(NO 3 ) 2 ·6H 2 O] was dissolved in 100ml of distilled water, stirred magnetically for 0.5 hour, and the solution formed was designated as solution A. Weigh 1.0 g of sodium diethyldithiocarbamate (DDTC) and dissolve it in 100 ml of distilled water, and magnetically stir for 0.5 hour, and the formed solution is recorded as B solution. The solution A was slowly added dropwise to the solution B, and stirred magnetically for 4.5 hours, and the formed solution was recorded as solution C. Stop stirring solution C, let it stand still for 12 hours, filter it with suction, wash it with distilled water and absolute ethanol three times, and dry it in a blast drying oven at 60°C for 12 hours to obtain a dry powder sample, which is a single-source molecular precursor.
[0040] (2) Preparation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com