Preparation method of hollow cobalt sulfide microsphere catalyst
A cobalt sulfide and catalyst technology, which is applied in cobalt sulfide, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of high operating temperature, complicated methods, harmful raw materials, etc., and achieve increased active area, good reproducibility, and long reaction time short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve 2 mmol of cobalt nitrate hexahydrate and 8 mmol of thioacetamide in 50 mL of water, and magnetically stir at room temperature for 10 min, while slowly dripping 1.4 mL of ethylenediamine solution, and continue stirring for 5 min. The obtained solution was sealed in a 70 mL stainless steel autoclave lined with polytetrafluoroethylene, and reacted at 180 °C for 16 h. Then it was cooled to room temperature, and the precipitate was taken out, and washed with deionized water and absolute ethanol three times at a speed of 1200 r, each time for 4 min. Finally, the product was vacuum-dried at 50 °C for 24 h to obtain cobalt sulfide with hollow microsphere structure.
Embodiment 2
[0030] Dissolve 2 mmol of cobalt nitrate hexahydrate and 8 mmol of thiourea in 50 mL of water, stir magnetically at room temperature for 10 min, and slowly add 2.6 mL of ethylenediamine solution dropwise, and continue stirring for 5 min. The obtained solution was sealed in a 70 mL stainless steel autoclave lined with polytetrafluoroethylene, and reacted at 180 °C for 20 h. Then it was cooled to room temperature, and the precipitate was taken out, and washed three times with deionized water and absolute ethanol at a speed of 1200r, each time for 4 min. Finally, the product was vacuum-dried at 50 °C for 24 h to obtain cobalt sulfide with hollow microsphere structure.
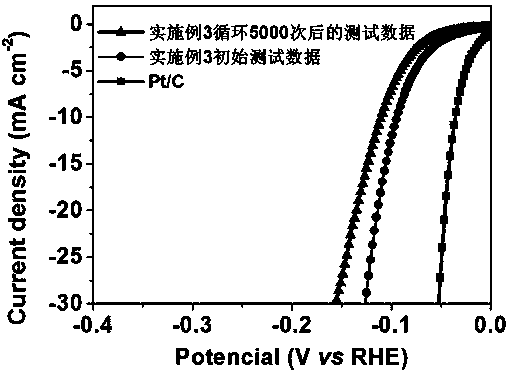
Embodiment 3
[0032] Dissolve 2 mmol of cobalt nitrate hexahydrate and 8 mmol of sulfur powder in 50 mL of water, stir magnetically at room temperature for 10 min, and slowly add 3.2 mL of ethylenediamine solution dropwise, and continue stirring for 5 min. The obtained solution was sealed in a 70 mL stainless steel autoclave lined with polytetrafluoroethylene, and reacted at 180 °C for 20 h. Then it was cooled to room temperature, and the precipitate was taken out, and washed three times with deionized water and absolute ethanol at a speed of 1200r, each time for 4 min. Finally, the product was vacuum-dried at 50 °C for 24 h to obtain cobalt sulfide with hollow microsphere structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Impedance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com