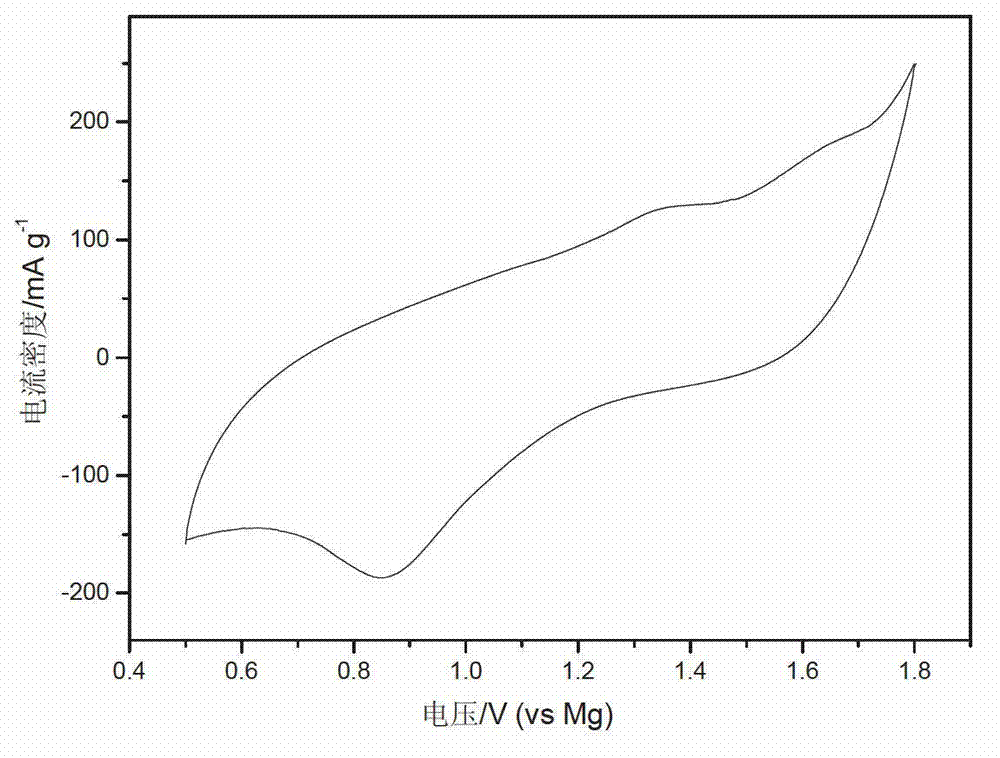
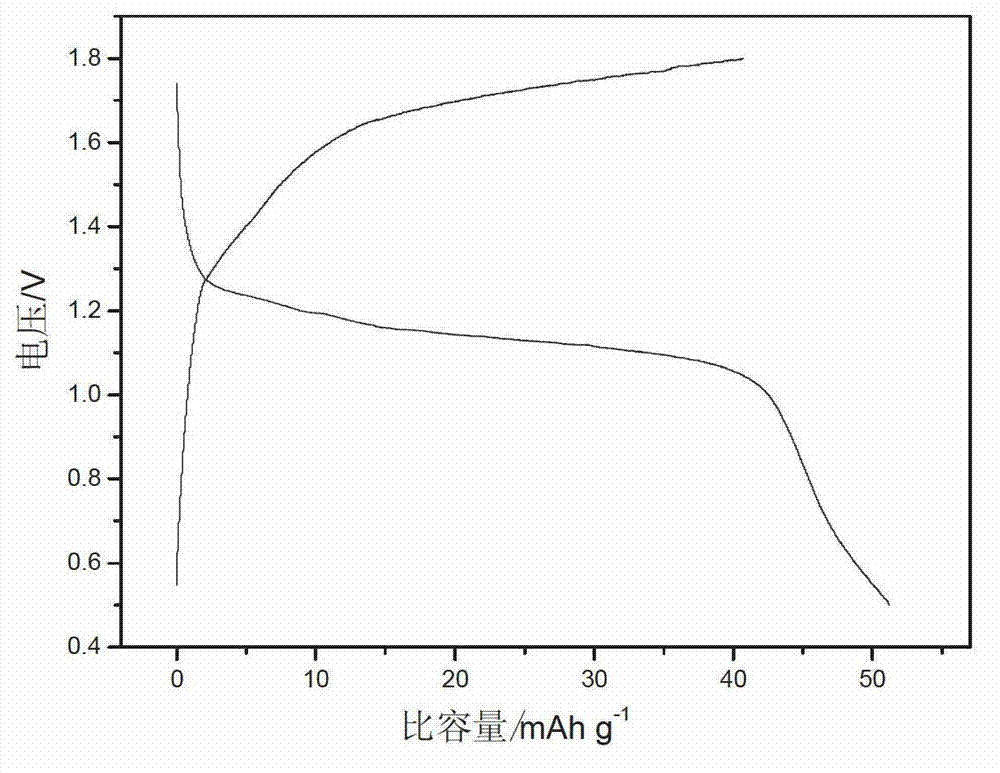
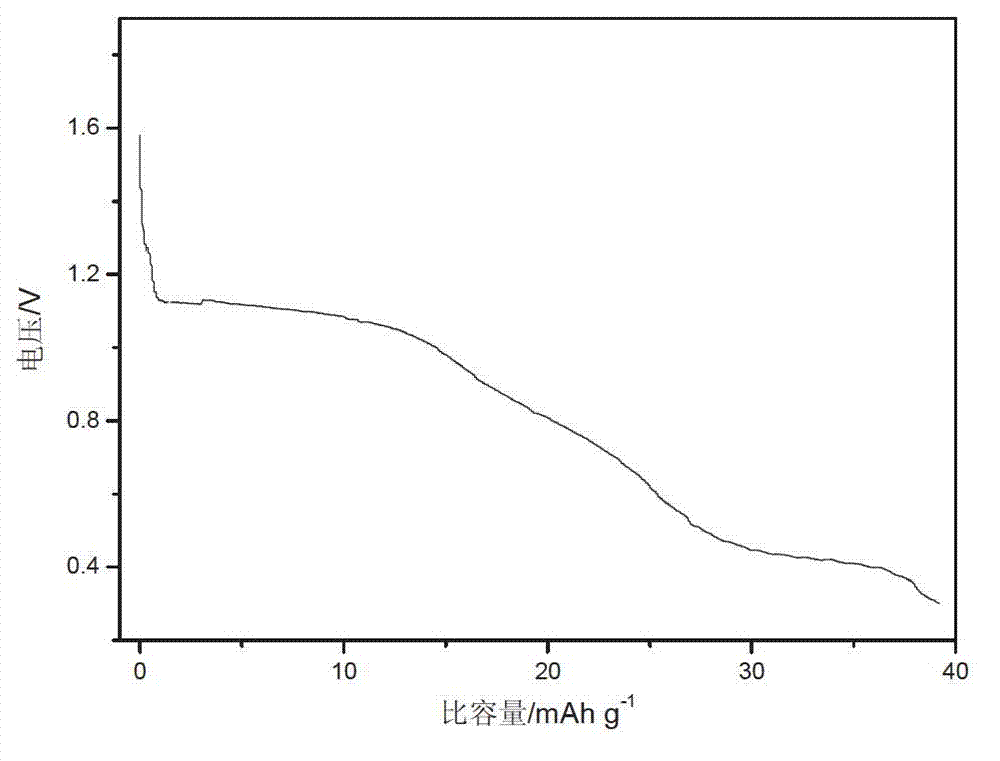
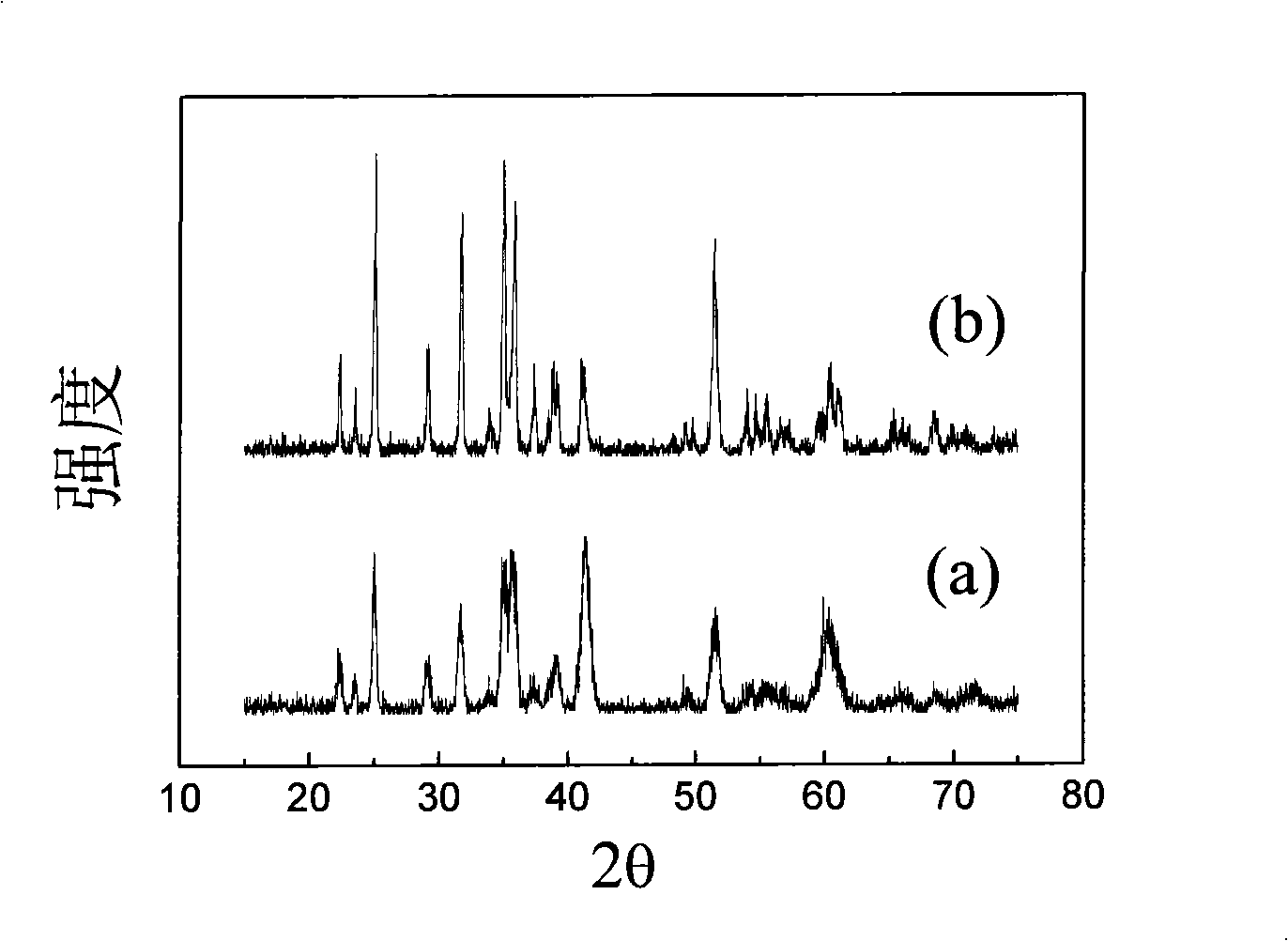
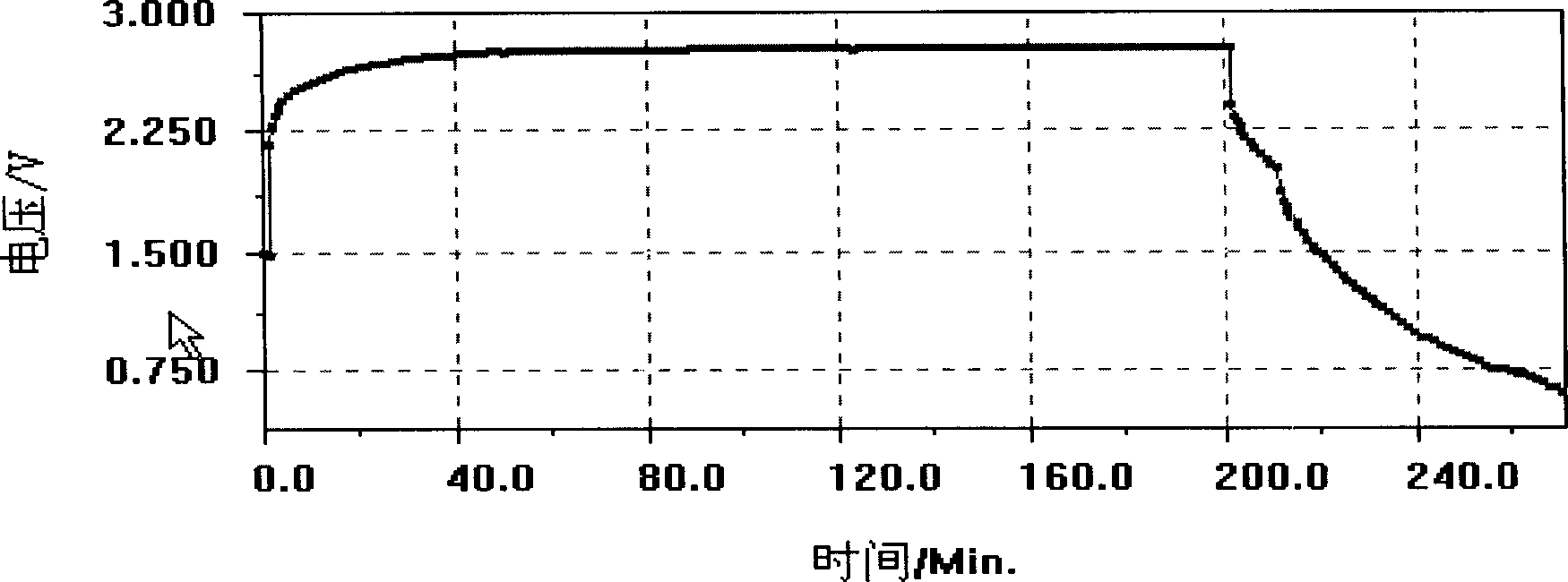
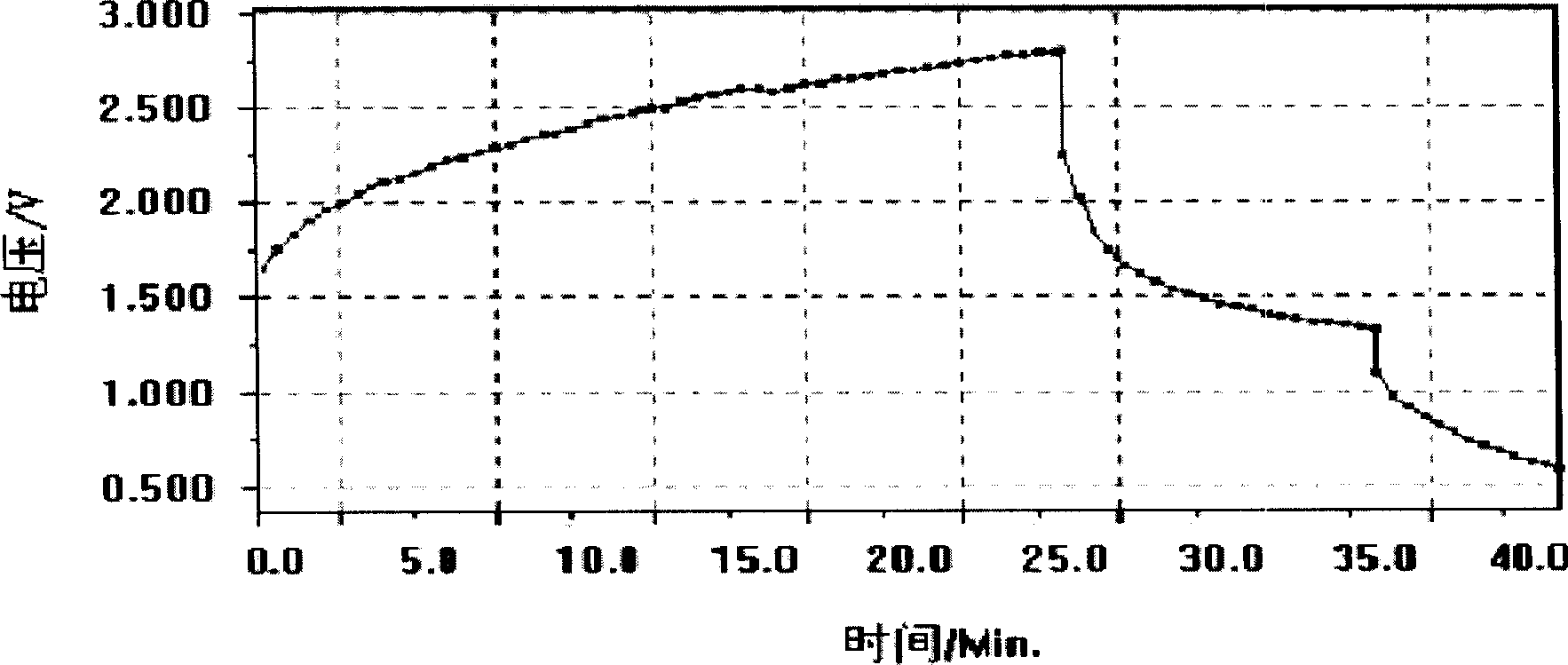
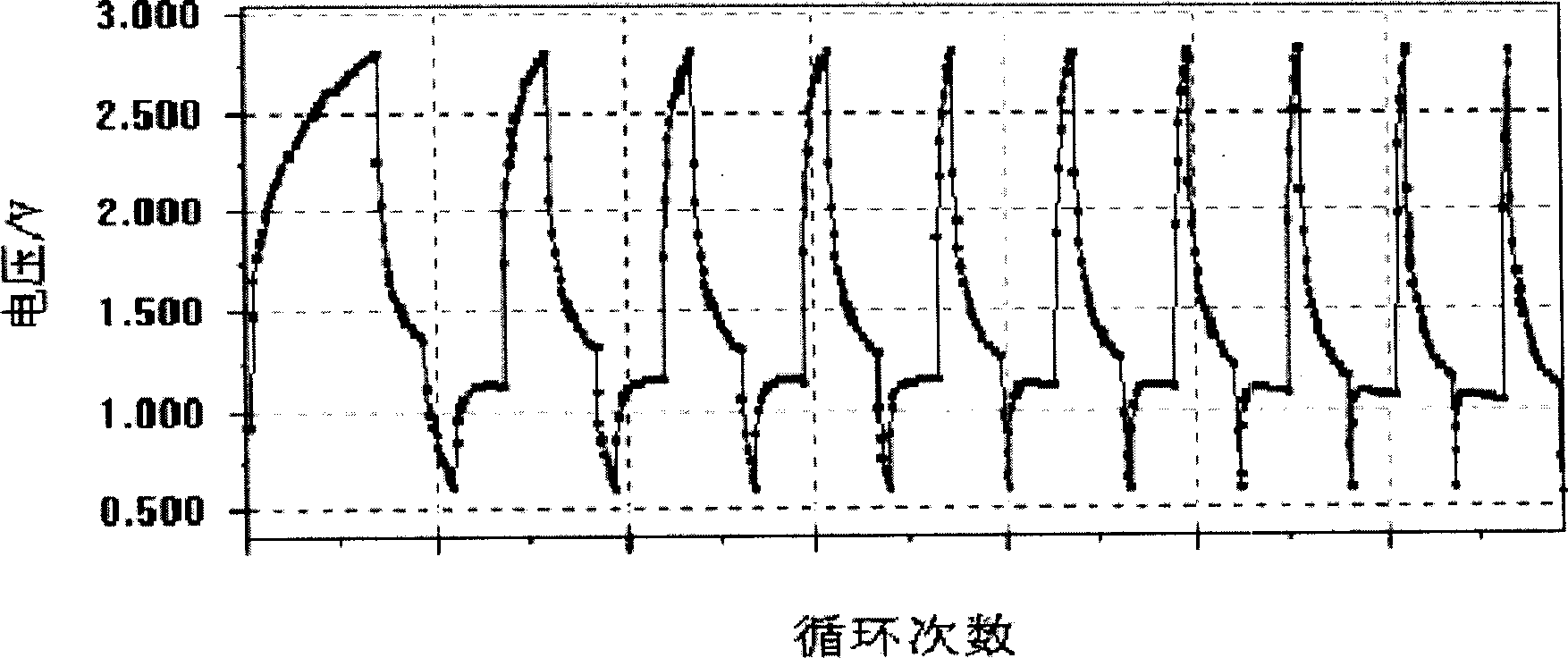
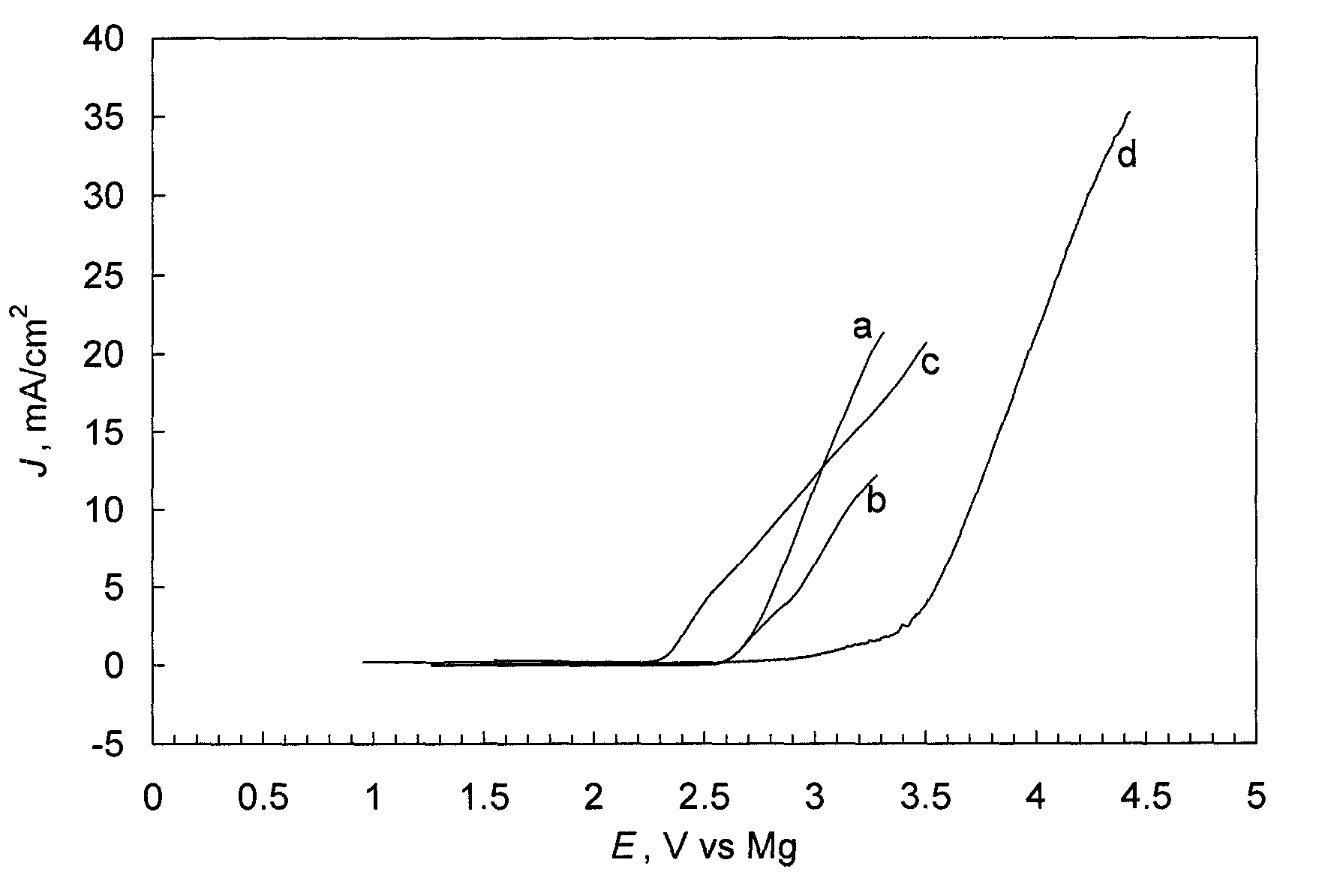
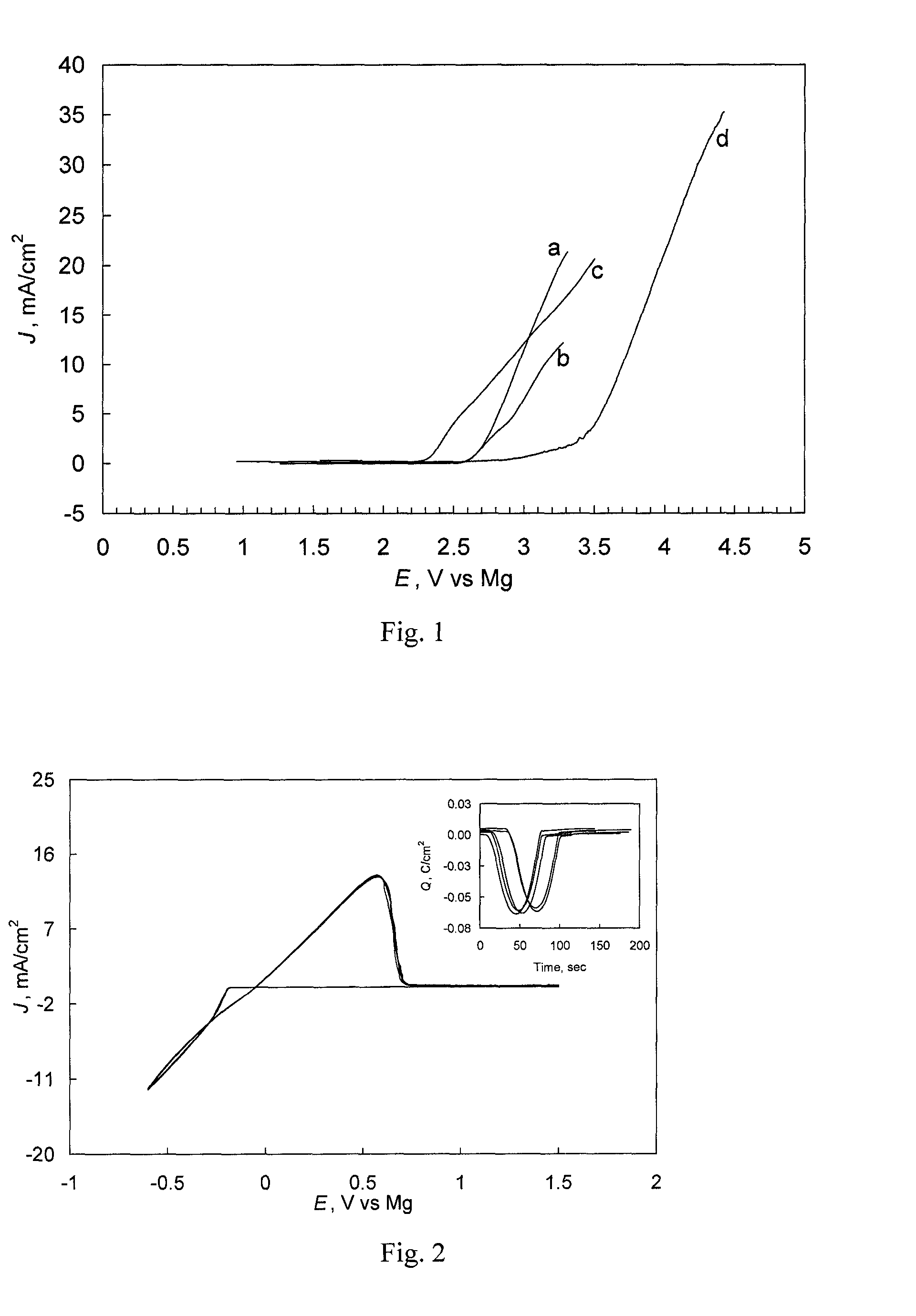
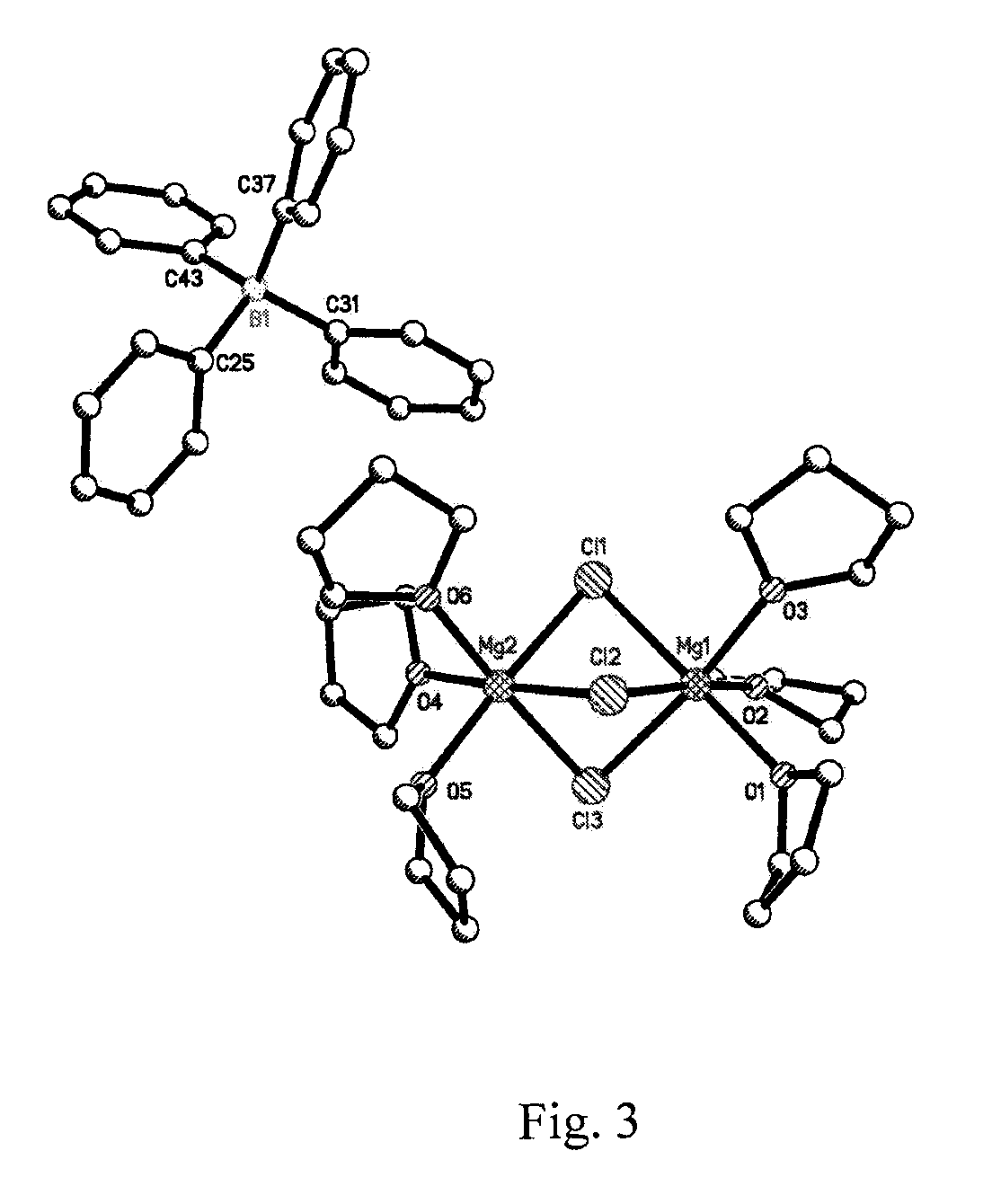
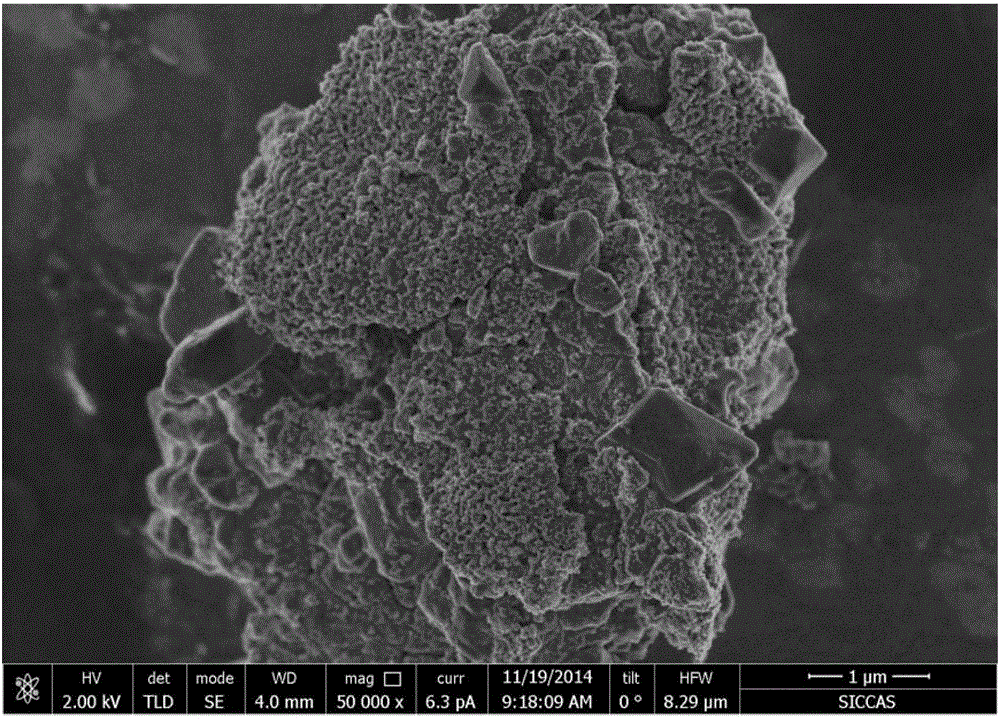
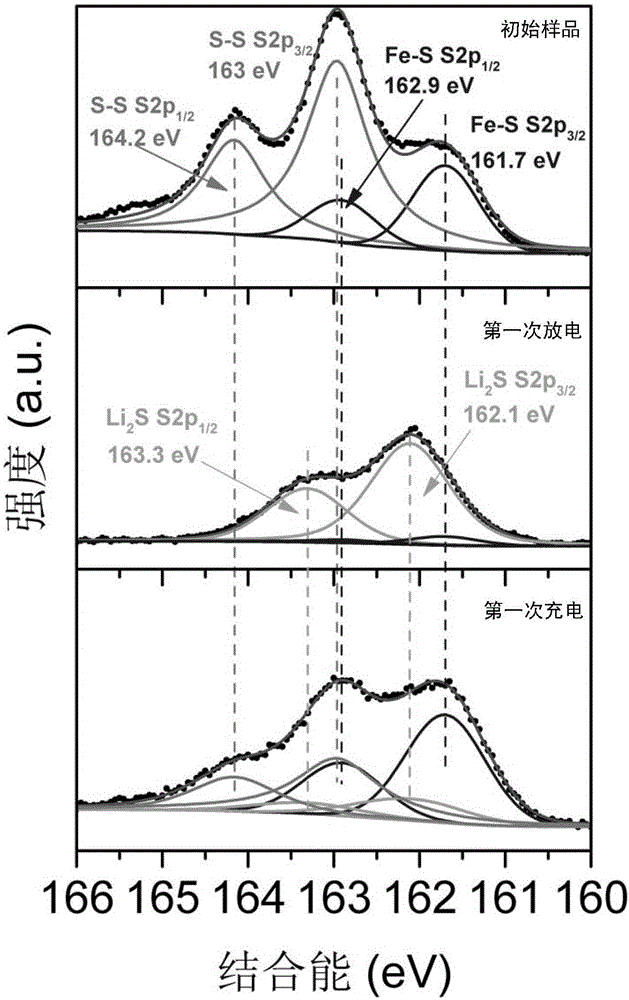
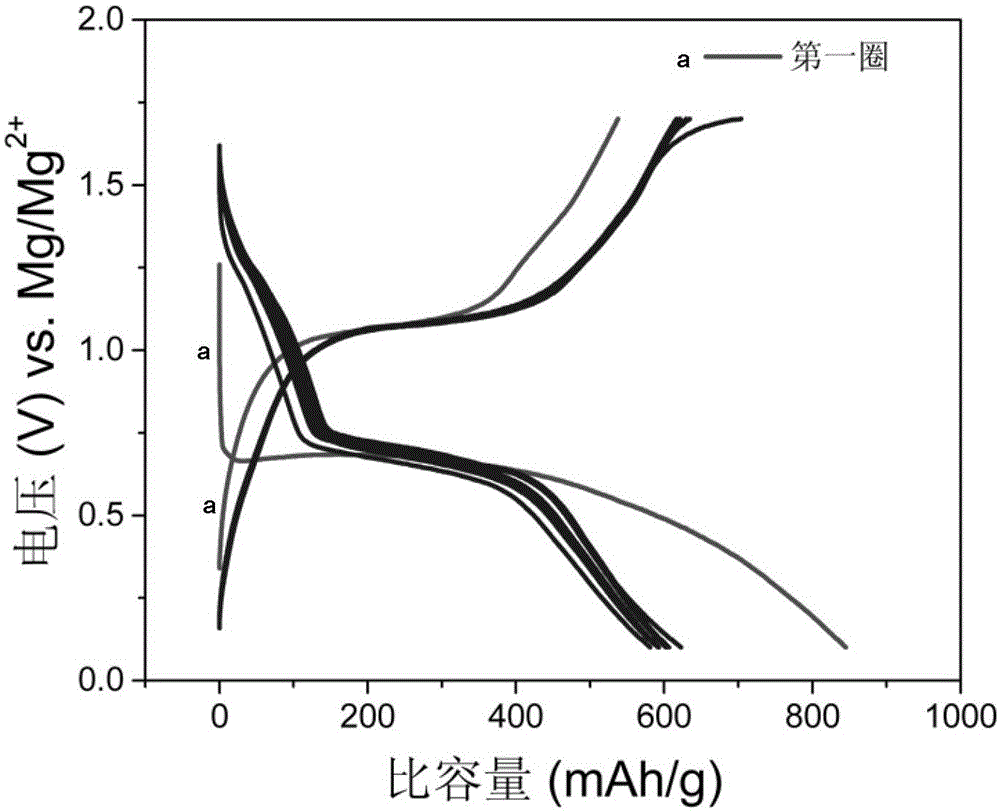
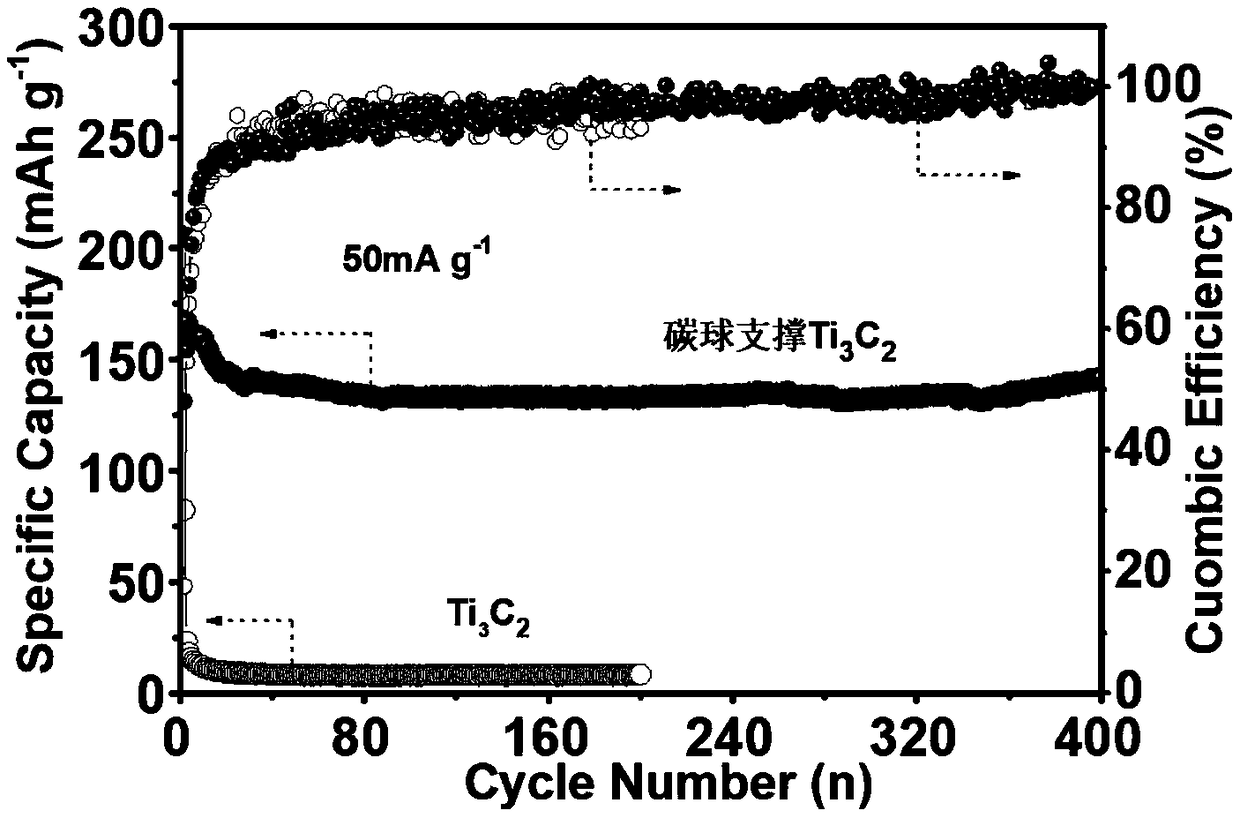
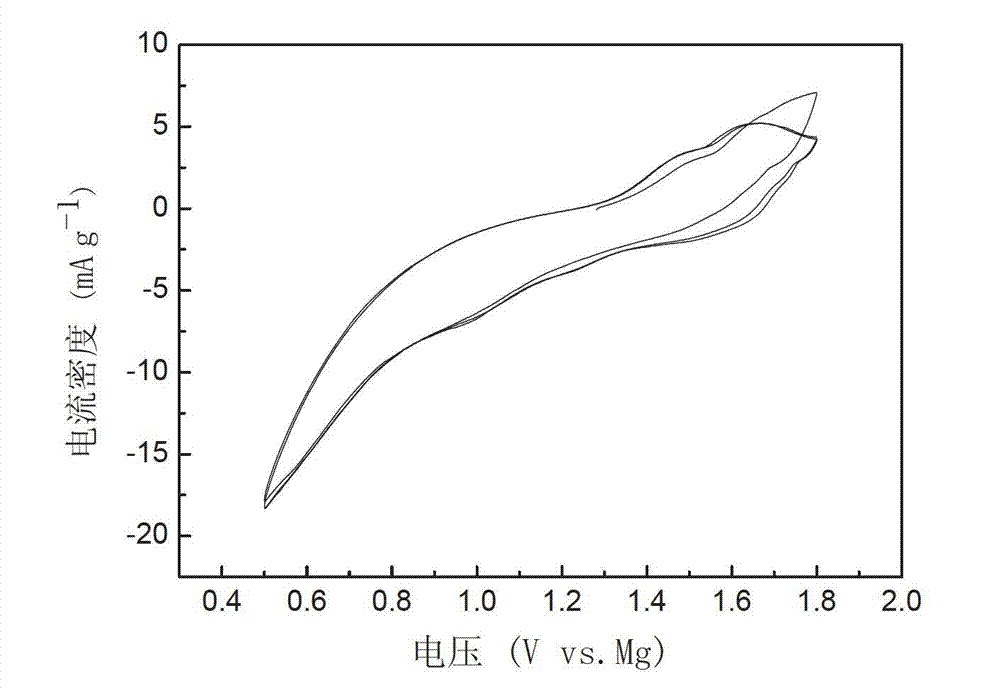
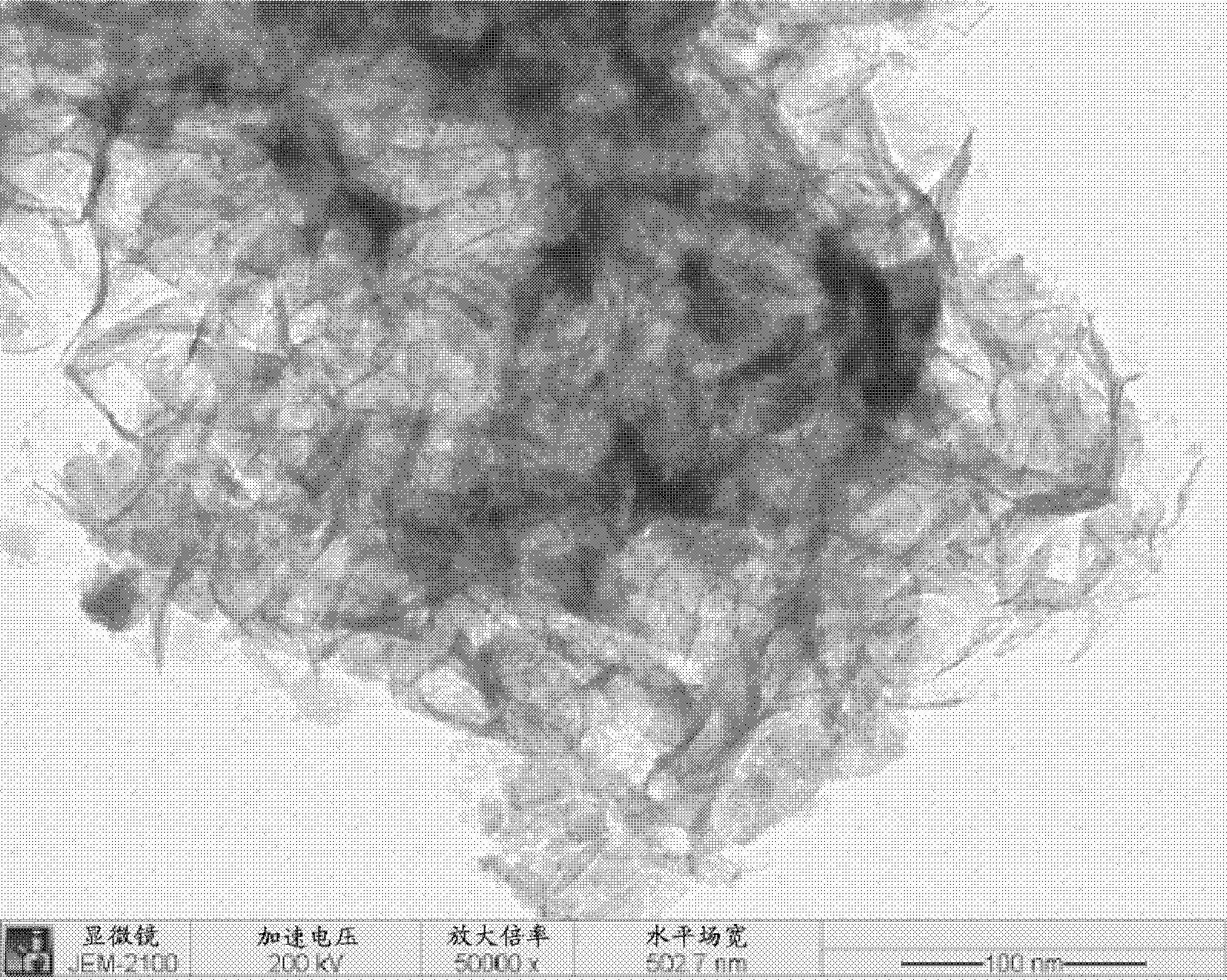Patents
Literature
204 results about "Magnesium battery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
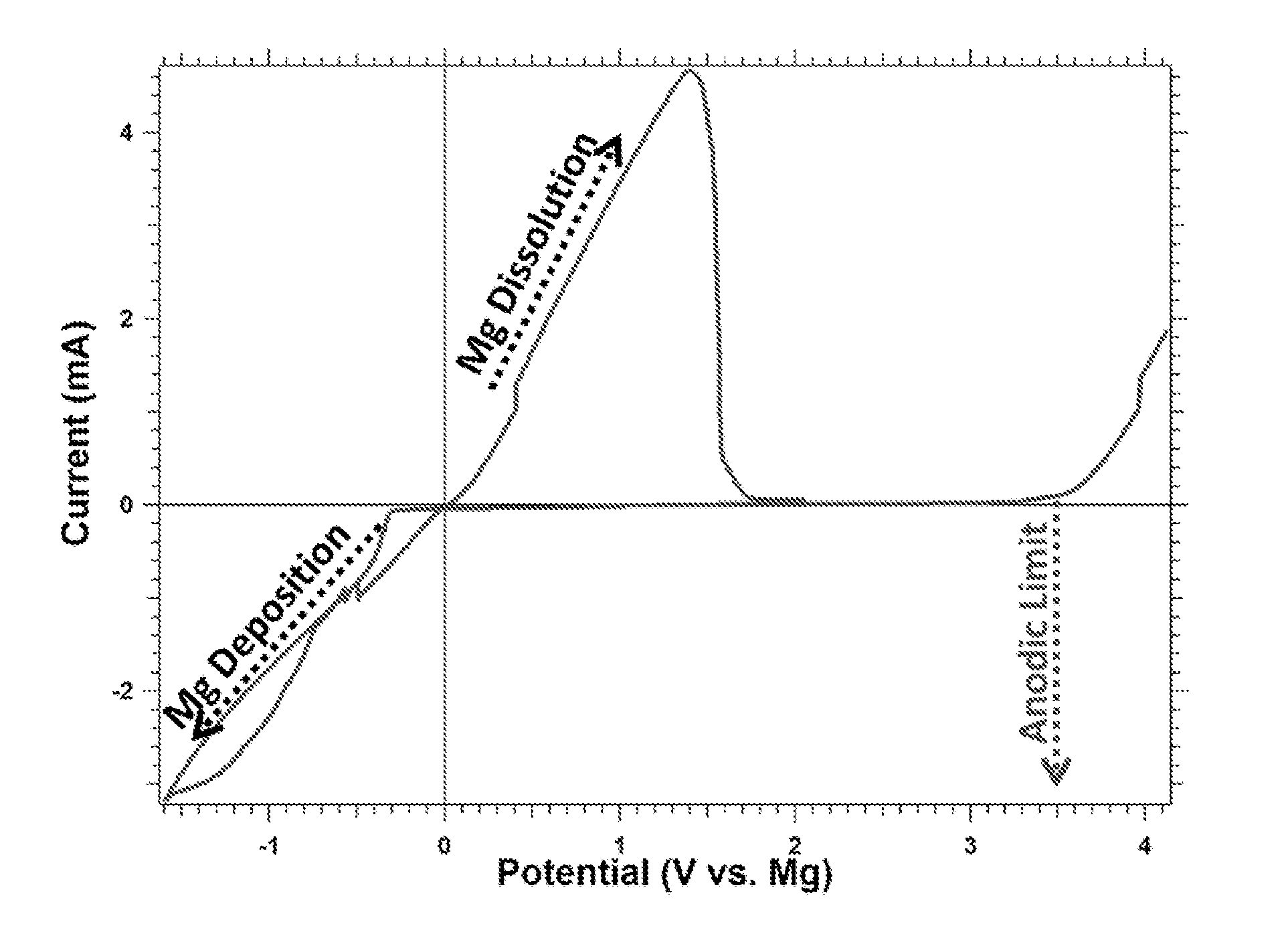
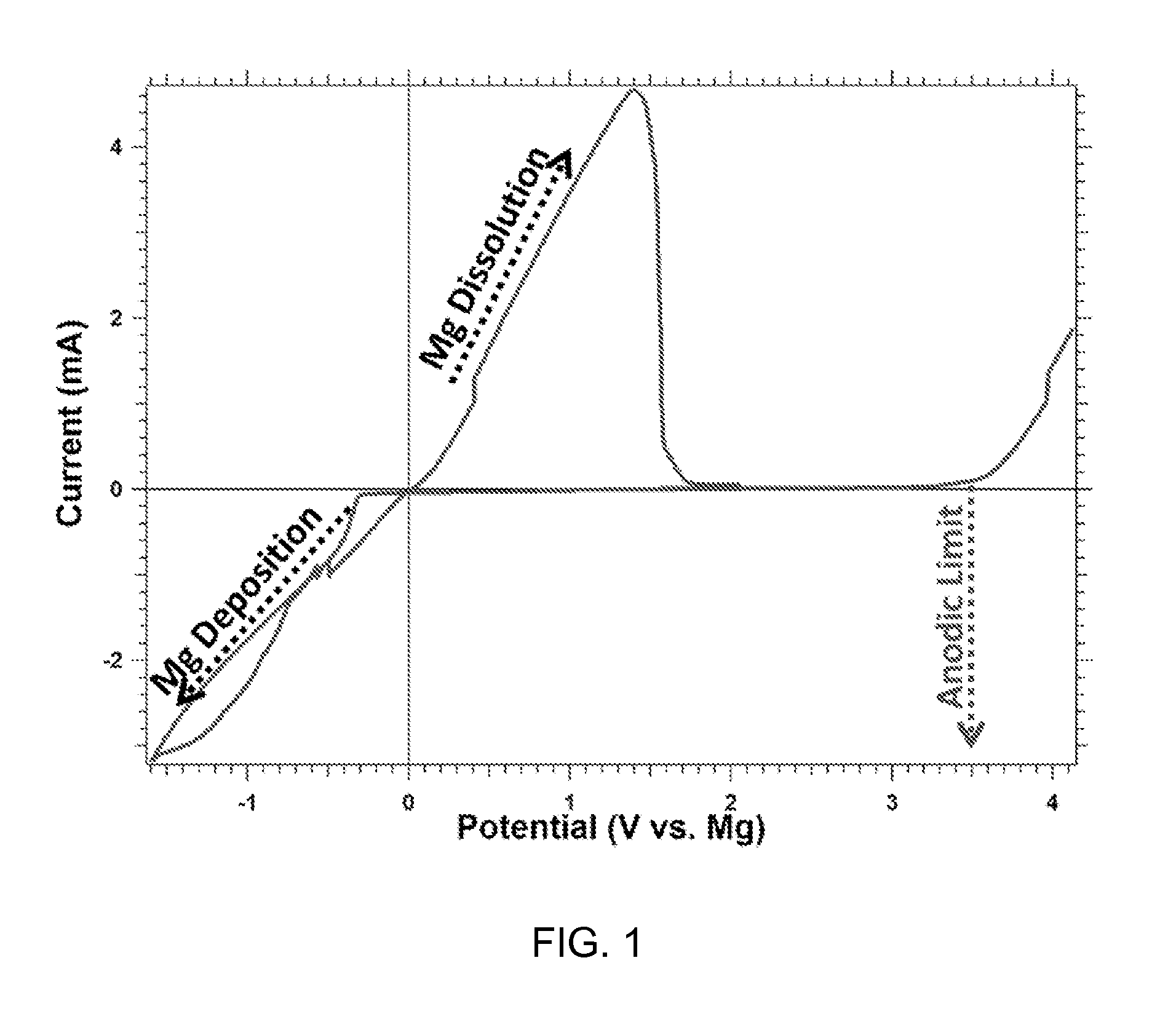
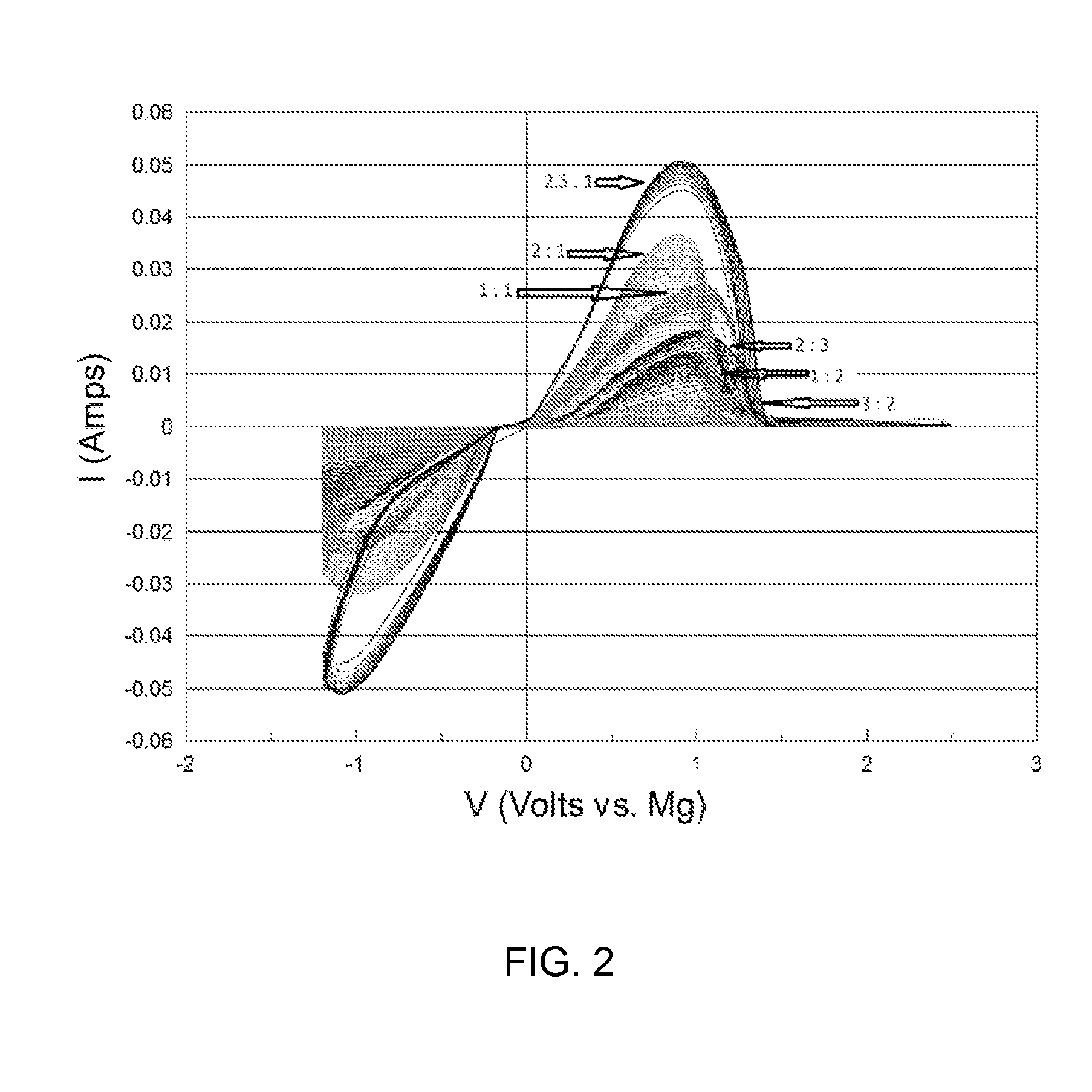
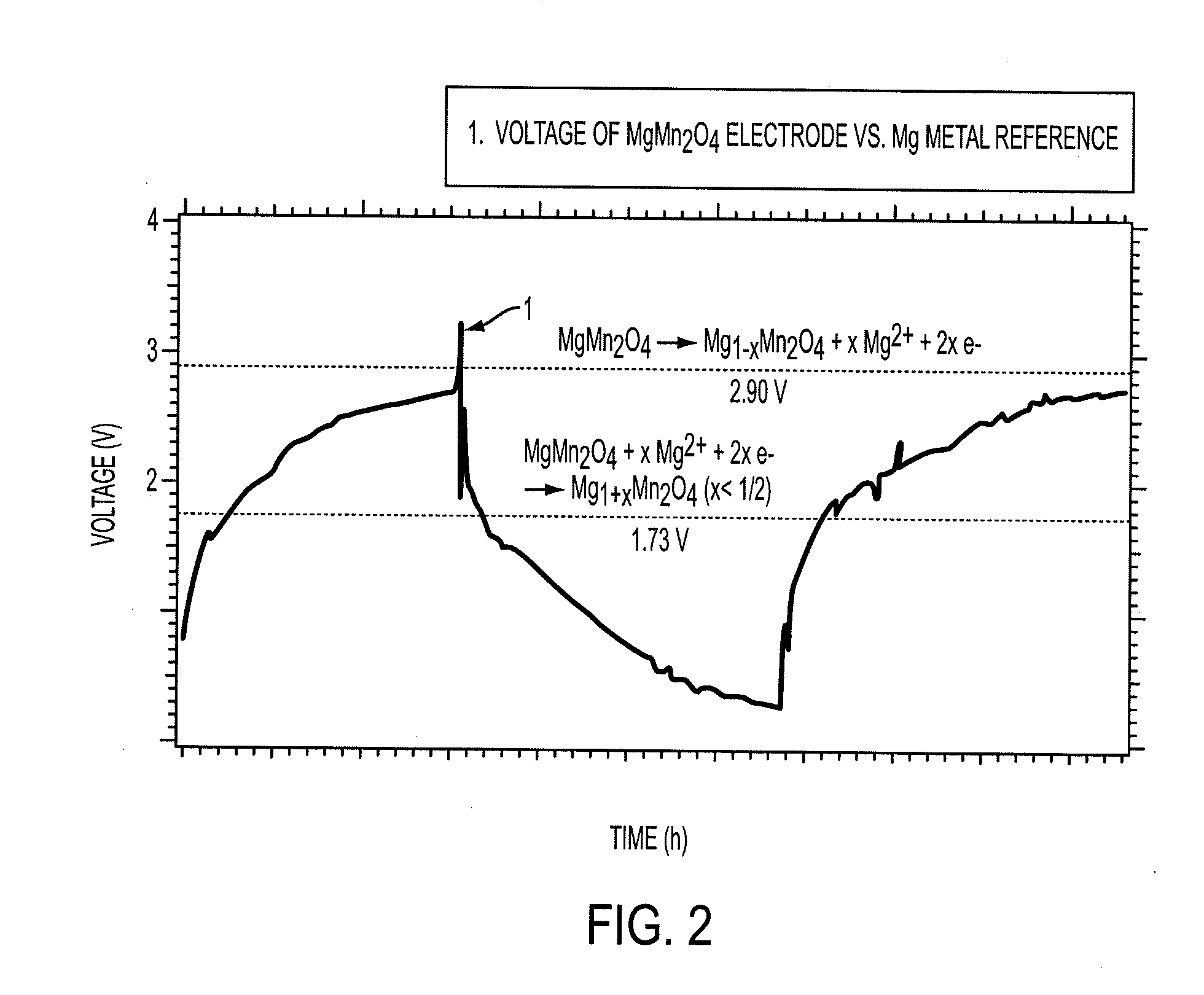
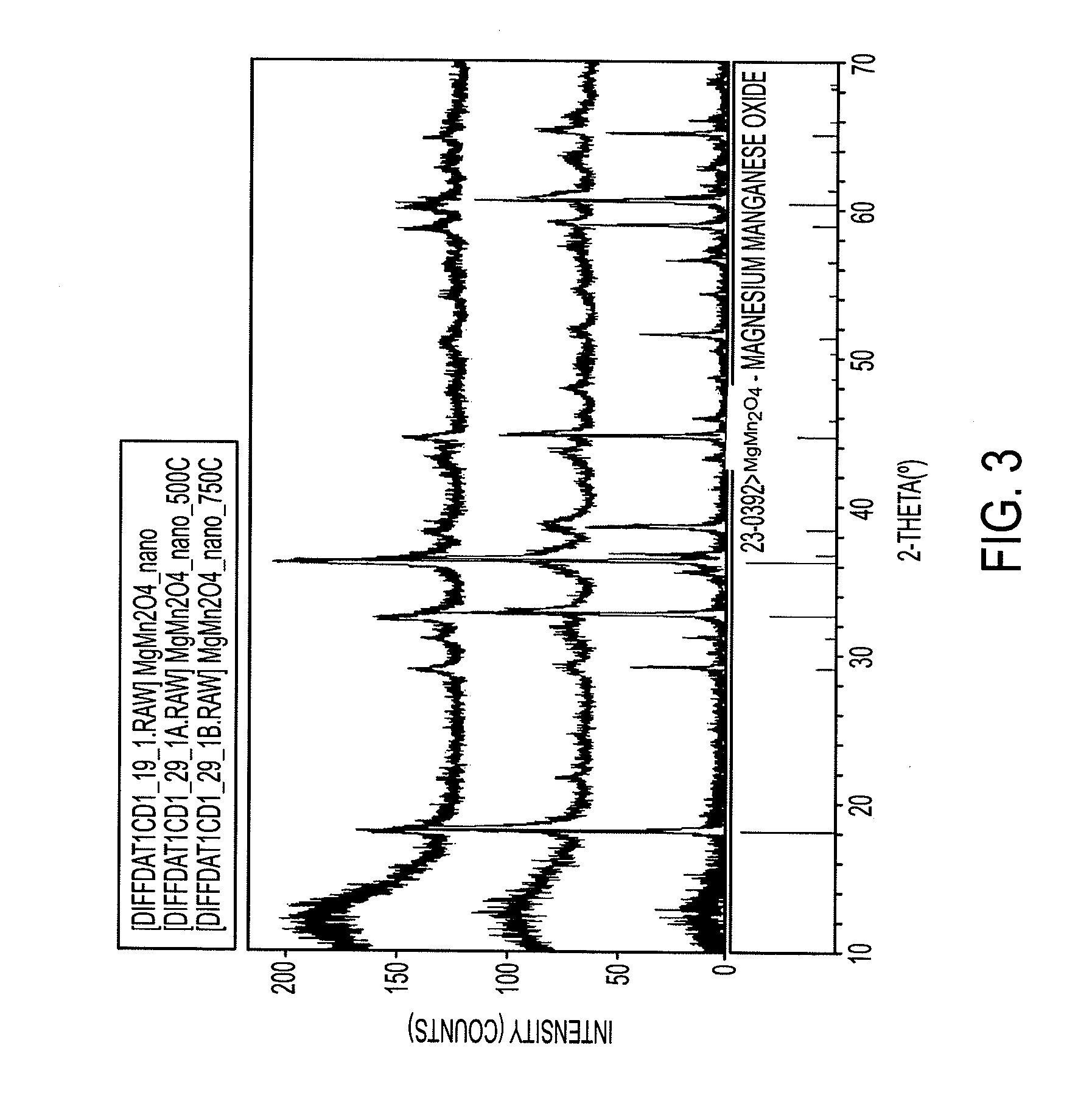
Magnesium batteries are batteries that utilize magnesium cations as the active charge transporting agent in solution and as the elemental anode of an electrochemical cell. Both non-rechargeable primary cell and rechargeable secondary cell chemistries have been investigated. Magnesium primary cell batteries have been commercialised and have found use as reserve and general use batteries.
Rechargeable magnesium ion cell components and assembly
InactiveUS20110159381A1Cost-effective fabricationHigh voltageSilver accumulatorsElectrode carriers/collectorsEngineeringMagnesium ion
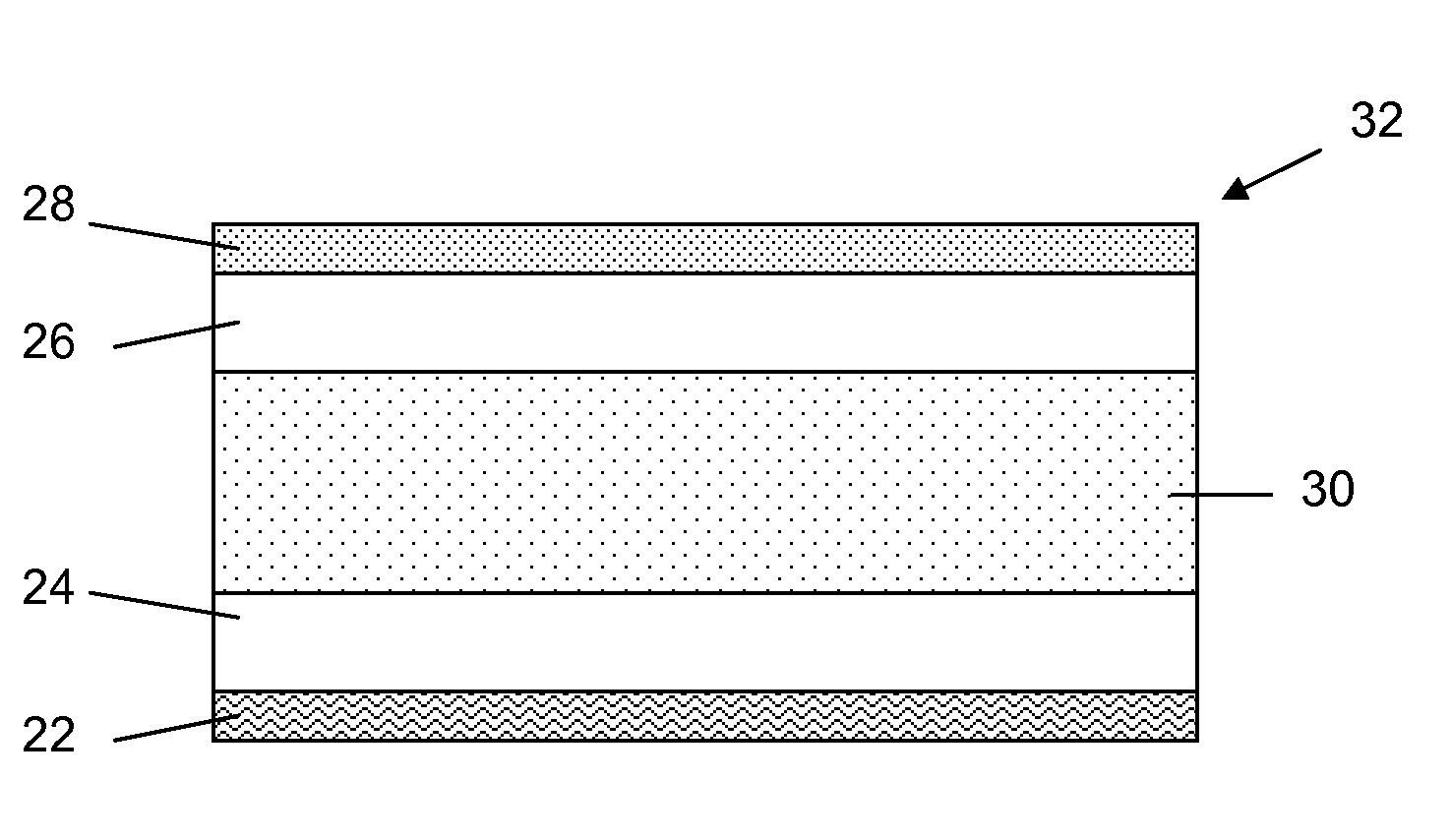
A magnesium battery electrode assembly is described, including a current collector comprising a carbonaceous material and an electrode layer comprising an electrode active material disposed on the current collector.
Owner:PELLION TECH
Rechargeable magnesium battery
ActiveUS20080182176A1Improved kineticsImprove conductivityMaterial nanotechnologyPhosphorus sulfur/selenium/tellurium compoundsElectrochemical windowCopper
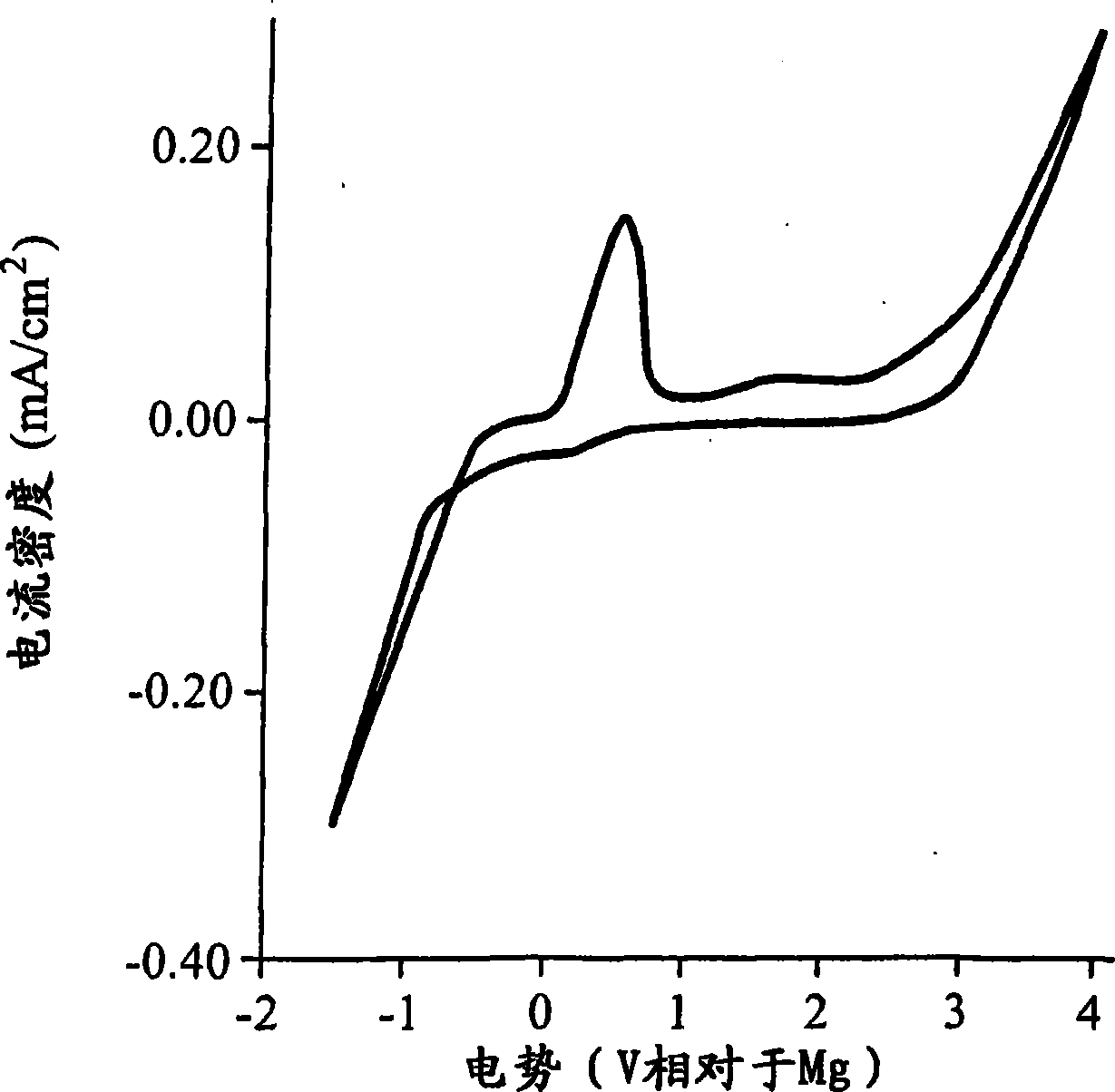
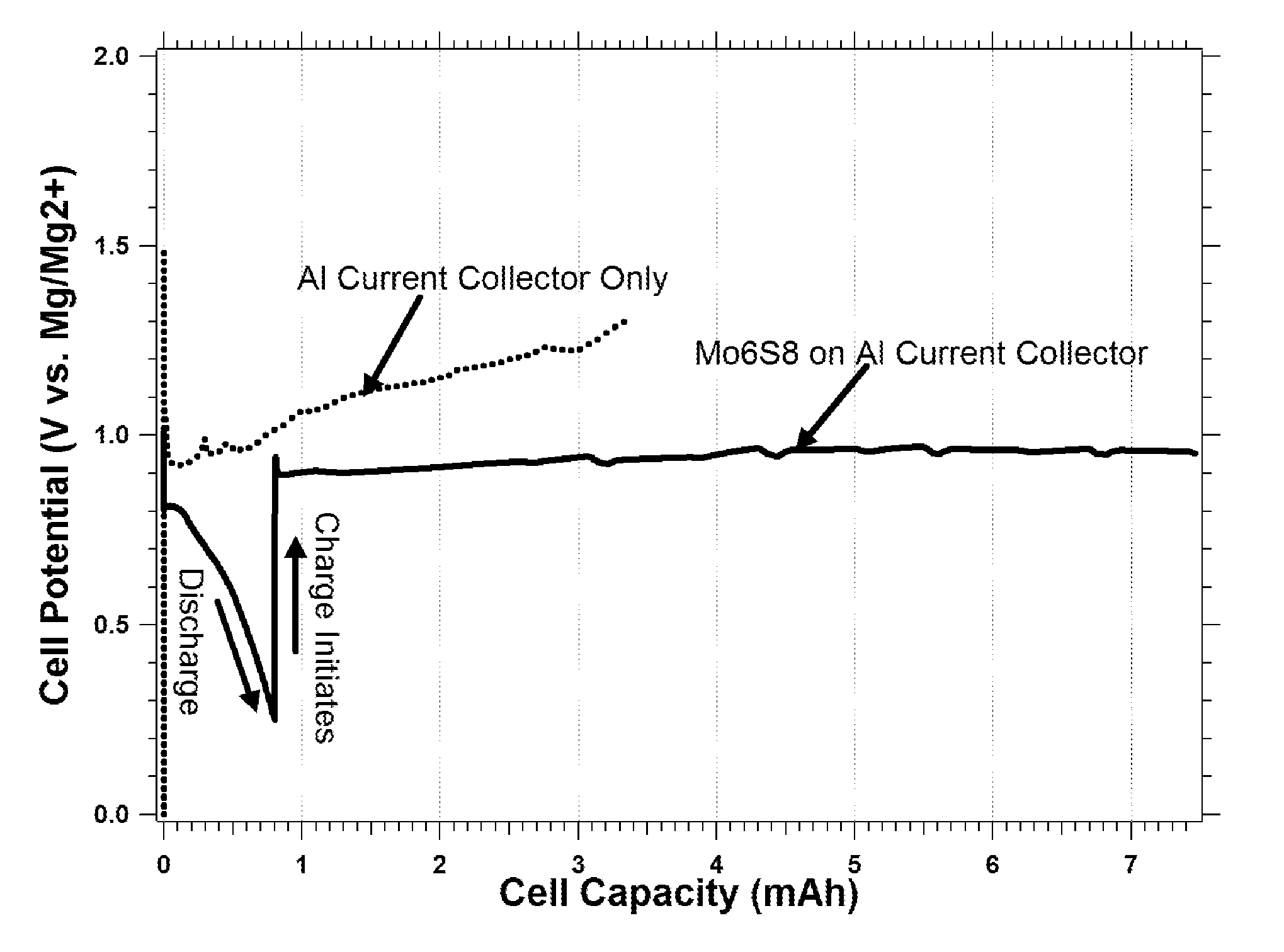
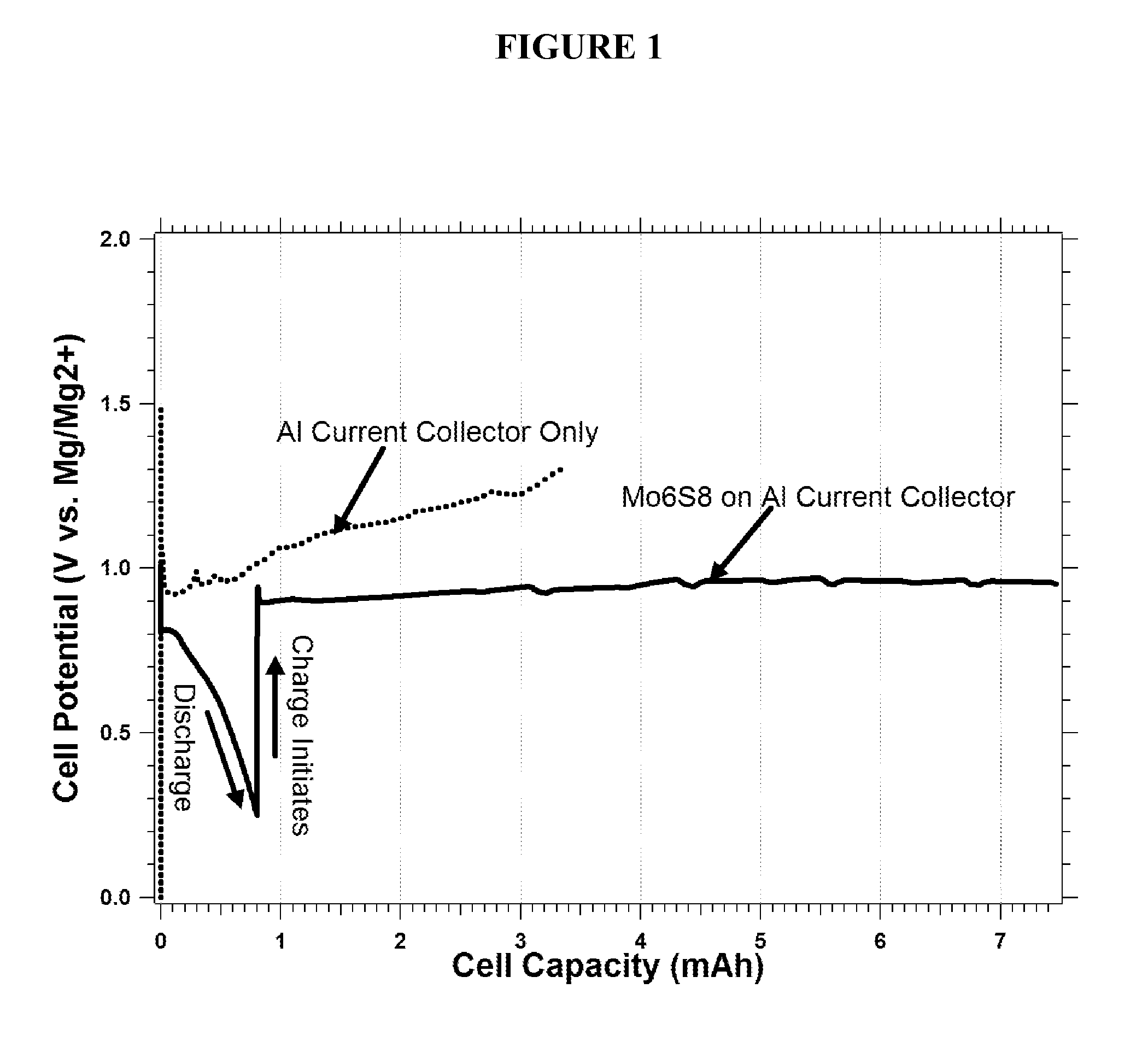
This invention generally relates to electrochemical cells utilizing magnesium anodes, new solutions and intercalation cathodes. The present invention is a new rechargeable magnesium battery based on magnesium metal as an anode material, a modified Chevrel phase as an intercalation cathode for magnesium ions and new electrolyte solution from which magnesium can be deposited reversibly, which have a very wide electrochemical window. The Chevrel phase compound is represented by the formula Mo6S8-YSeY in which y is higher than 0 and lower than 2 or by the formula MXMo6S8 in which M is selected from the group comprising of copper (Cu), nickel (Ni), silver (Ag) and / or any other transition metal; further wherein x is higher than 0 and lower than 2.
Owner:BAR ILAN UNIV
Electrolyte for magnesium battery
InactiveUS20130034780A1Safe handlingReduce environmental impactCell electrodesOrganic electrolyte cellsMagnesium saltGrignard reagent
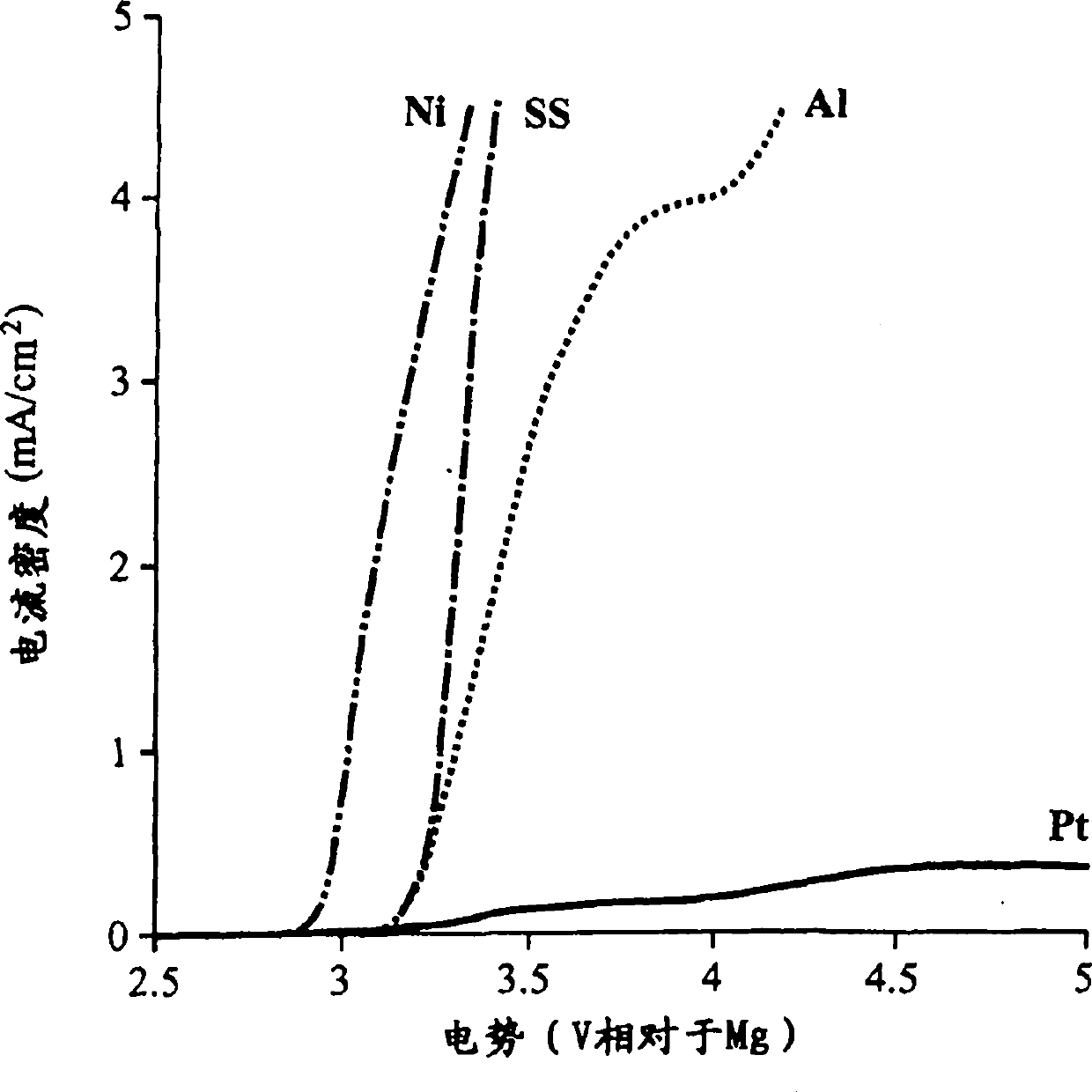
A magnesium battery, having an anode containing magnesium; a cathode stable to a voltage of at least 2.6 V relative to a magnesium reference; and an electrolyte containing an electrochemically active magnesium salt obtained by reaction of a Grignard reagent or Hauser base with a boron compound of formula BR3 is provided. The electrolyte is stable to 2.6 E.V. vs. Mg in the presence of stainless steel.
Owner:TOYOTA JIDOSHA KK
Magnesium ion-containing nonaqueous electrolytic solution and method for manufacturing the same, and electrochemical device
ActiveUS20100136438A1Easy to makeRaw material is easyAlkaline accumulatorsCell electrodesDimethylaluminum chlorideAlloy
Owner:MURATA MFG CO LTD
High voltage rechargeable magnesium batteries having a non-aqueous electrolyte
InactiveUS20130252112A1Improve conductivityImprove solubilityOrganic electrolyte cellsSecondary cellsGrignard reagentMetallic materials
A rechargable magnesium battery having an non-aqueous electrolyte is provided. The properties of the electrolyte include high conductivity, high Coulombic efficiency, and an electrochemical window that can exceed 3.5 V vs. Mg / Mg+2. The use of the electrolyte promotes the electrochemical deposition and dissolution of Mg without the use of any Grignard reagents, other organometallic materials, tetraphenyl borate, or tetrachloroaluminate derived anions. Other Mg-containing electrolyte systems that are expected to be suitable for use in secondary batteries are also described.
Owner:PELLION TECH
High-performance rechargeable magnesium battery and manufacturing method thereof
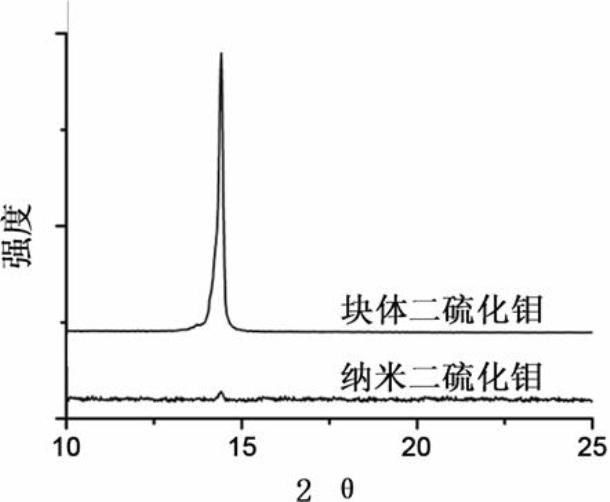
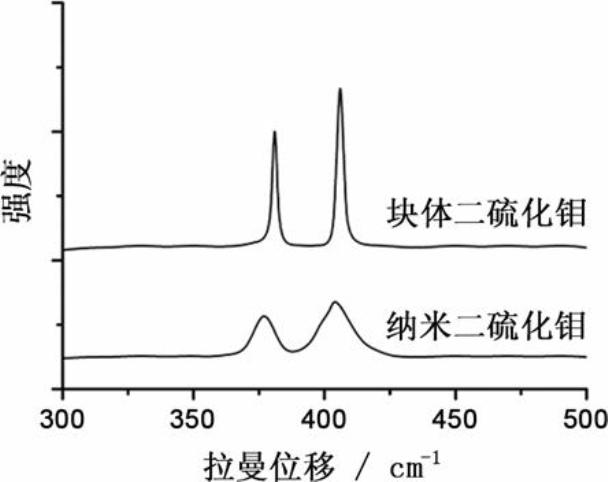
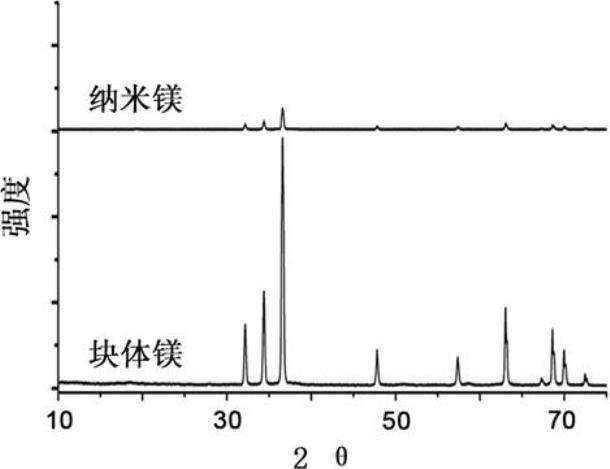
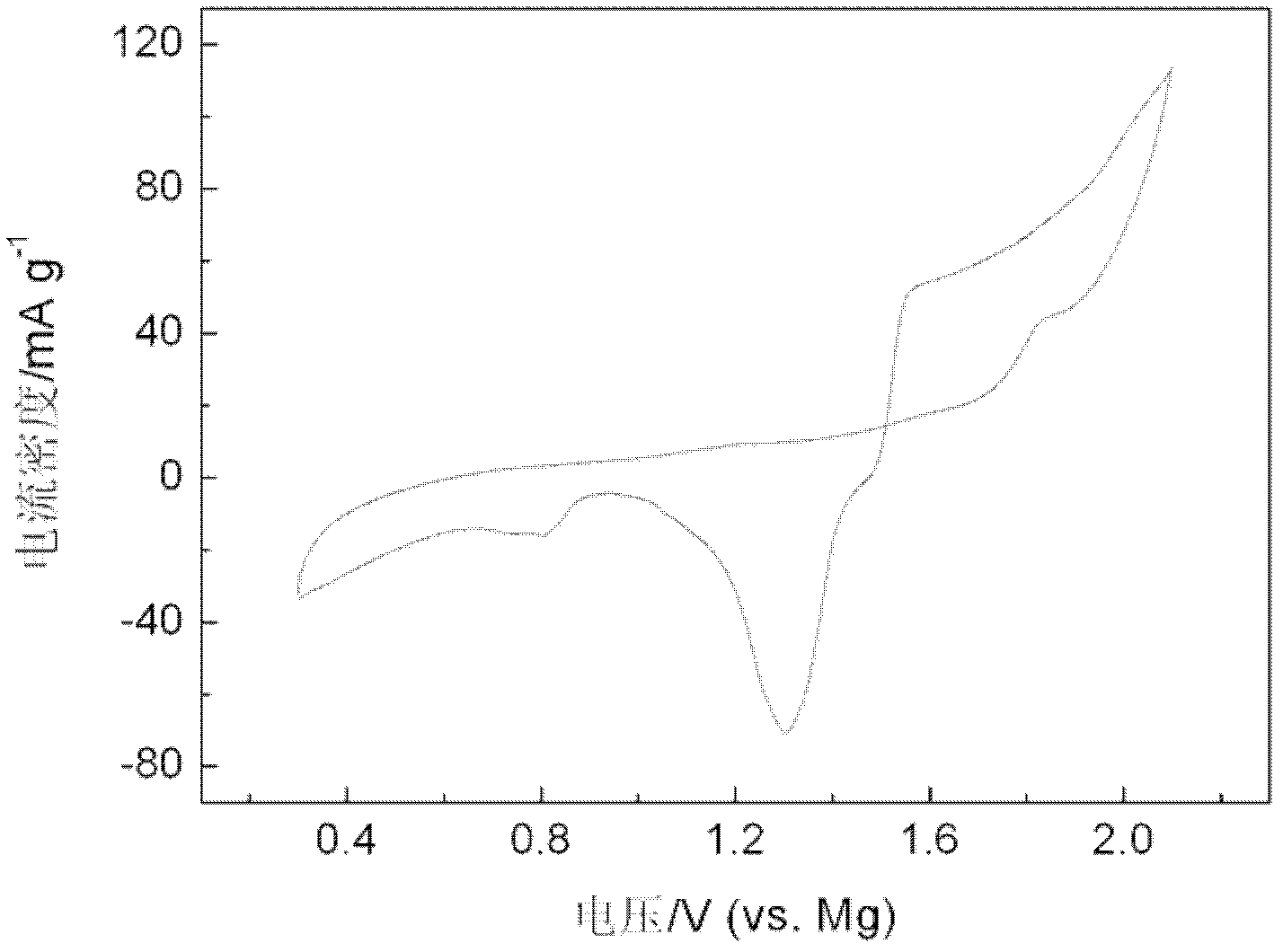
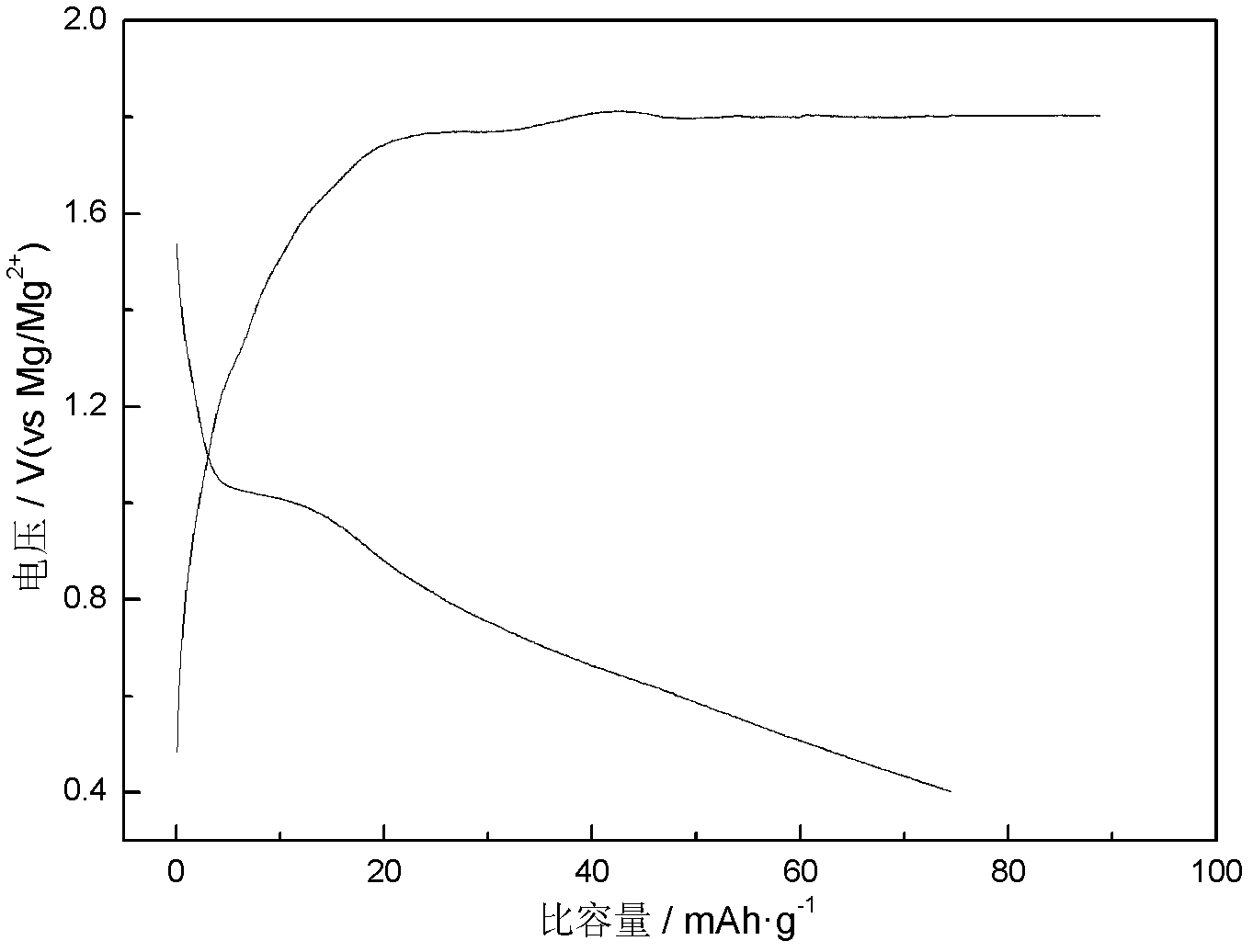
ActiveCN102024996AMild conditions for material preparationHigh specific capacityFinal product manufactureCell electrodesGrignard reagentPolypropylene
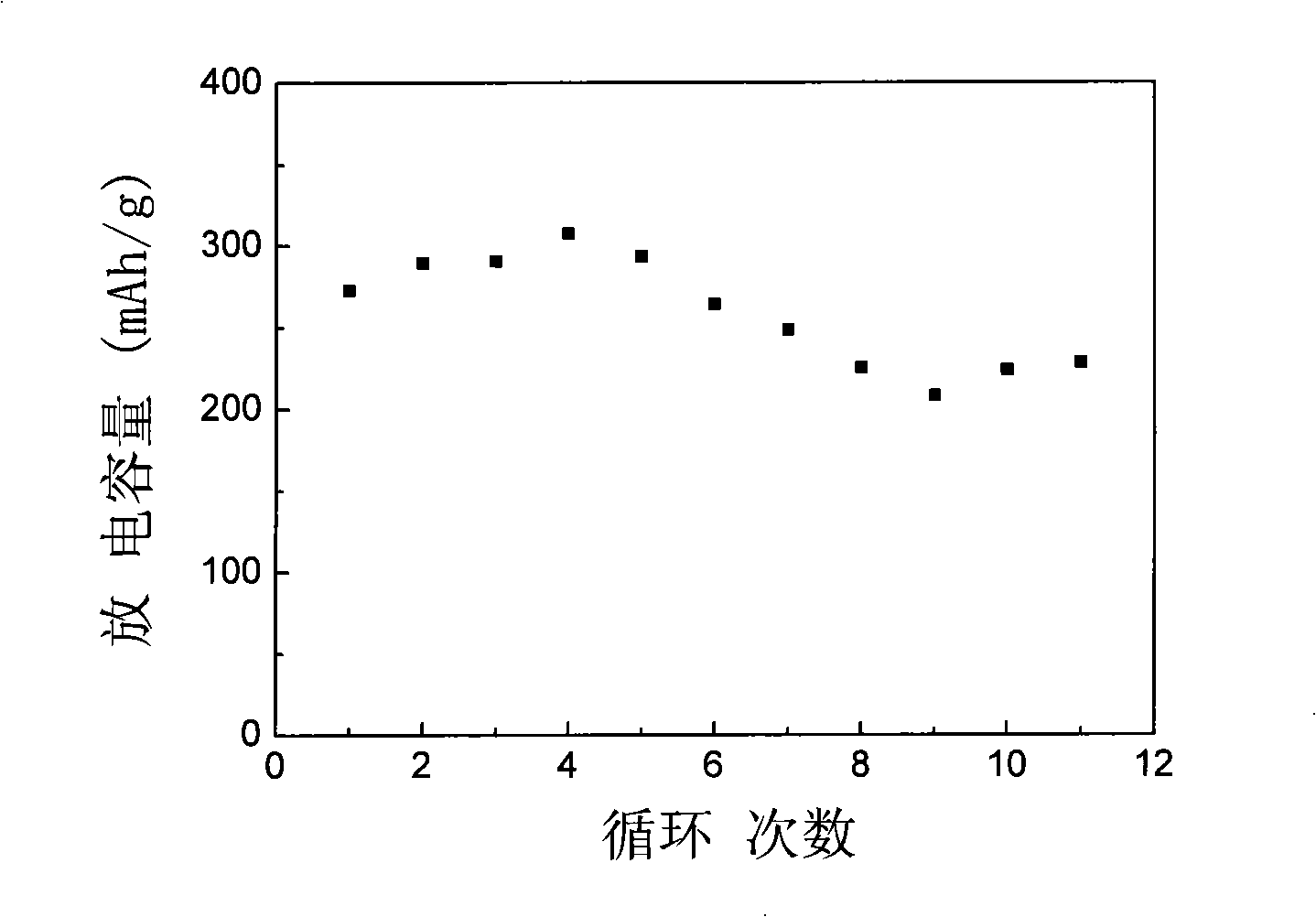
The invention discloses a high-performance rechargeable magnesium battery consisting of a positive plate, a negative plate, a diaphragm and an electrolyte. The positive plate is made from highly stripped nano-supramoly which is of highly erosive structure, wherein the average number of the layers of the nano-supramoly is not more than 4, and the average thickness is not more than 3nm. The negative plate is made from grain-shaped nanometer-level magnesium or nanometer-micrometer level composite magnesium, and the average grain diameter is 1-10nm. The diaphragm is a three-layer film made from polythene, polypropylene and polyethylene. The electrolyte is made from a tetrahydrofuran solution of Grignard reagent derivate. The invention has the advantages that: the rechargeable battery has gentler material preparation conditions (from the room temperature to 150 DEG C), larger specific capacity (170mAhg<-1>), higher operating voltage (1.8V), better circulation performance (still keeping 95%of initial capacity after circulating for 50 periods) and the like compared with the reported magnesium secondary battery system, and the rechargeable battery can be applied to the next generation large-scale energy storage batteries.
Owner:NANKAI UNIV
Rechargeable magnesium battery taking oxygen-containing organic matter as cathode material, and preparation method thereof
InactiveCN102683744AEasy to manufactureIncrease discharge voltage platformCell electrodesSecondary cellsQuinoneOrganic matter
The invention discloses a rechargeable magnesium battery taking oxygen-containing organic matter as a cathode material, and a preparation method of the rechargeable magnesium battery, wherein the oxygen-containing organic matter comprises the components of quinones, phenols, anhydrides derivatives and a compound containing nitro and oxygen radical. The battery and the method have the advantages of being simple in preparation technology, easy to process, environment-friendly and high in regeneration. The rechargeable magnesium battery prepared by the invention has the advantages of being relatively good in structural stability and circulation stability, rich in raw materials, safe in system and the like.
Owner:SHANGHAI JIAO TONG UNIV
Electrode materials for magnesium batteries
InactiveUS20120219859A1Stable maintenanceReduce spreadSilver accumulatorsAlkaline accumulator electrodesMaterials scienceMagnesium battery
Owner:PELLION TECH
Application method of binary metal sulfides in chargeable magnesium battery
InactiveCN102969501AUnderstand the purposeLearn about featuresCell electrodesFinal product manufactureNickel sulfideCobalt sulfide
The invention discloses an application method of binary metal sulfides in a chargeable magnesium battery. The binary metal sulfides comprise nickel sulfide, manganese sulfide, cobalt sulfide, ferric sulfide, tin sulfide, tungsten sulfide, zinc sulfide, vanadic sulfide and the like. The preparation method for preparing the chargeable magnesium battery with the binary metal sulfides as an anode material specifically comprises the steps of: grinding the binary metal sulfides; adding a conductive agent and a bonding agent into the binary metal sulfides, and agitating uniformly and coating on a current collector; placing the current collector in an oven to dry, punching into a pole piece by a punch, tabletting and placing into a vacuum oven to dry so as to obtain the anode material; and using magnesium as a cathode, adding an electrolyte and assembling the chargeable magnesium battery. The chargeable magnesium battery prepared by the method provided by the invention has the advantages of simpleness in preparation, abundance in materials, low cost, and easiness in mass production. The chargeable magnesium battery has a great advantage as a large energy storing battery and has a good application prospect as a green energy resource.
Owner:SHANGHAI JIAO TONG UNIV
Preparation method for anode material manganese magnesium silicate of rechargeable magnesium cell
InactiveCN101320806AImprove crystal structureImprove electrochemical activityElectrode manufacturing processesChemical/physical/physico-chemical processesCapacitanceManganese
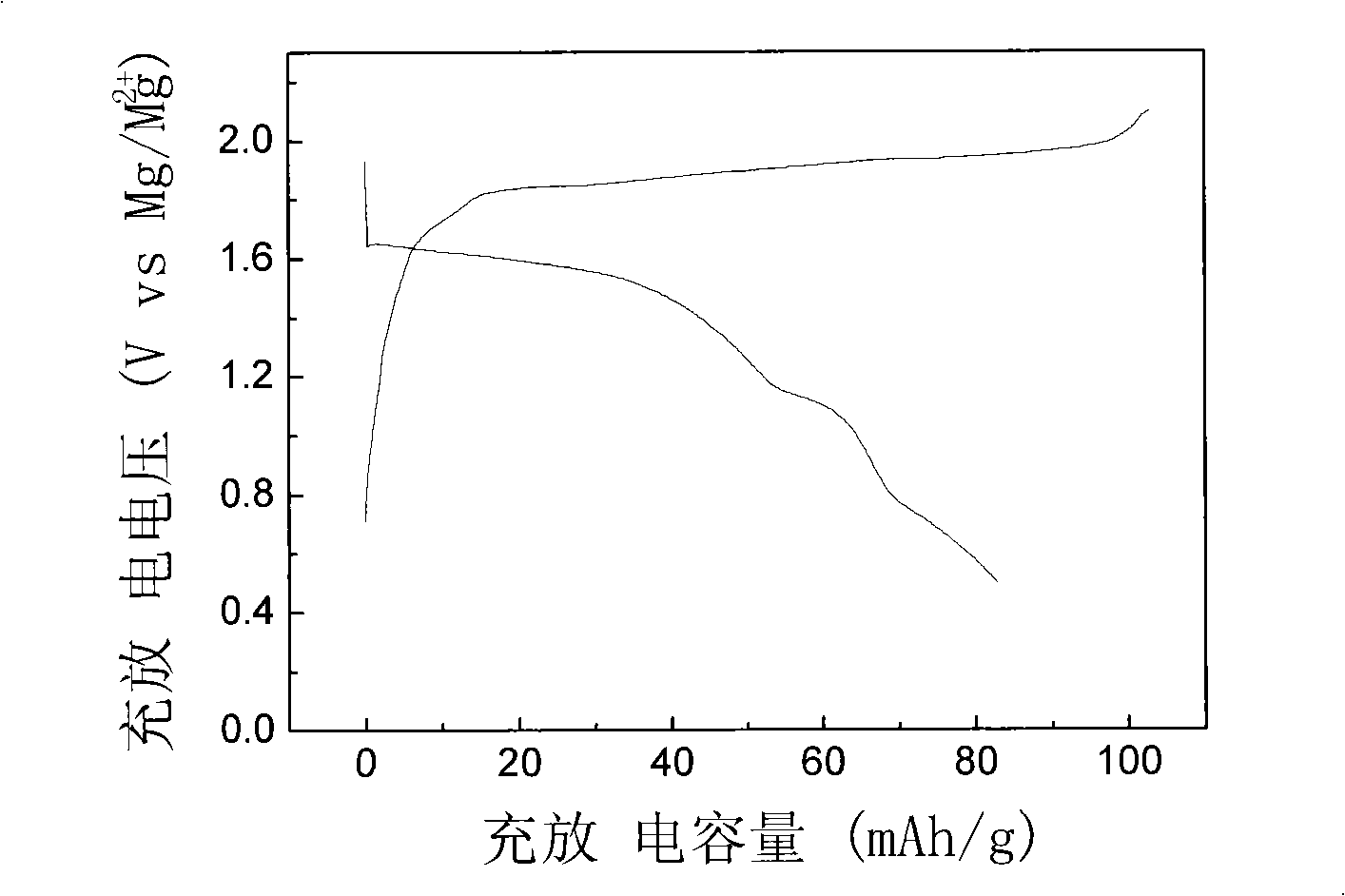
The invention discloses a production method for manganous / magnesium silicate of chargeable magnesium battery positive pole material, which is the manganous / magnesium silicate of chargeable magnesium battery positive pole material using molten salts as reaction medium and having the characteristics of quickening up reaction speed, shortening reaction cycle, simplifying synthesis course, reducing synthesis cost, small synthesis particle size and symmetrical distribution of the particles. The material exhibits remarkable electrochemical charge and discharge effects, the steady discharge platform works up to 1.6V and 1.1V(vs.Mg / Mg<2+>); on the charge and discharge conditions of C / 20 current density, the discharge capacitance can reach 289.3mAh*g<-1>(theoretical capacitance is 92%). In contrast to the relatively ideal positive pole material Mo3S4 of the current chargeable magnesium battery, the manganous / magnesium silicate positive pole material produced by the molten salt process has the advantages of simple production, large capacitance, high discharge voltage platform and the like.
Owner:SHANGHAI JIAO TONG UNIV
Positive active material for rechargeable magnesium battery and rechargeable magnesium battery
InactiveCN102723479ARealize charging and dischargingIncrease energy densityCell electrodesSecondary cellsCharge and dischargeMaterials science
The invention aims to provide a positive active material for a rechargeable magnesium battery and the rechargeable magnesium battery. Charge and discharge can be realized when the positive active material is used in the rechargeable magnesium battery. In addition, the positive active material can improve characteristics of the rechargeable magnesium battery. The positive active material for the rechargeable magnesium battery is represented by the molecular formula MgMSiO4, wherein M contains at least one element selected from Co, Ni and Fe.
Owner:SHOEI CHEM IND CO LTD
Chargeable magnesium battery
InactiveCN1411083ACharge and discharge balanceLow priceFinal product manufactureCell electrodesBattery chargeAlloy
This invention relates to the manufacture of secondary batteries charged by Mg including electrode materials, electrolytic material and their preparation method in which the negative is made of Mg alloy with component of MgMxMy(o<x, y<0.5) alloy above binary (M is Ni, Cu, Ti, Co, Si, B etc.), the positive is made of nanometer degree M2Co2O4(O<z<2, O<t<3) or MoS2 and the liquid electrolyte is metal organic compound Mg(ZnBuCl2)2. The said battery system has balance of charge / discharge with open voltage of about 2.0 V.
Owner:NANKAI UNIV
Rechargeable magnesium ion cell components and assembly
InactiveUS20130115521A1Improve chemical inertnessImproves anodic stabilityElectrode carriers/collectorsOrganic electrolyte cellsCorrosion reactionMagnesium ion
A magnesium battery electrode assembly is described, including a current collector comprising a metal, an overlayer material on the metal and an electrode layer comprising an electrode active material disposed on the current collector. The overlayer material passivates the metal, or inhibits a corrosion reaction that would occur between the metal and an electrolyte in the absence of the overlayer material.
Owner:PELLION TECH
Electrode materials for magnesium batteries
InactiveUS20120219856A1Stable maintenanceReduce spreadElectrode carriers/collectorsSecondary cellsMaterials scienceMagnesium battery
Owner:PELLION TECH
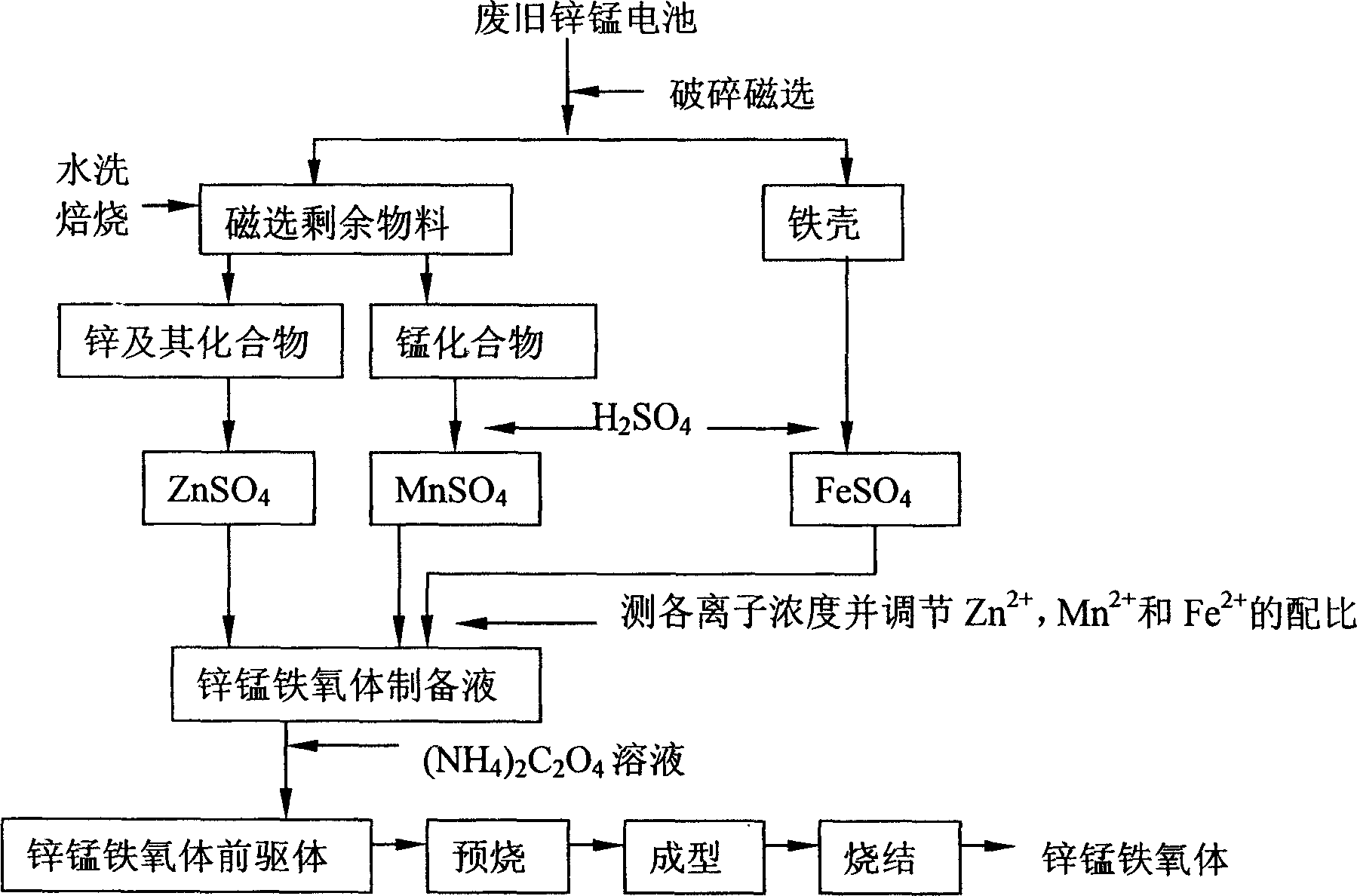
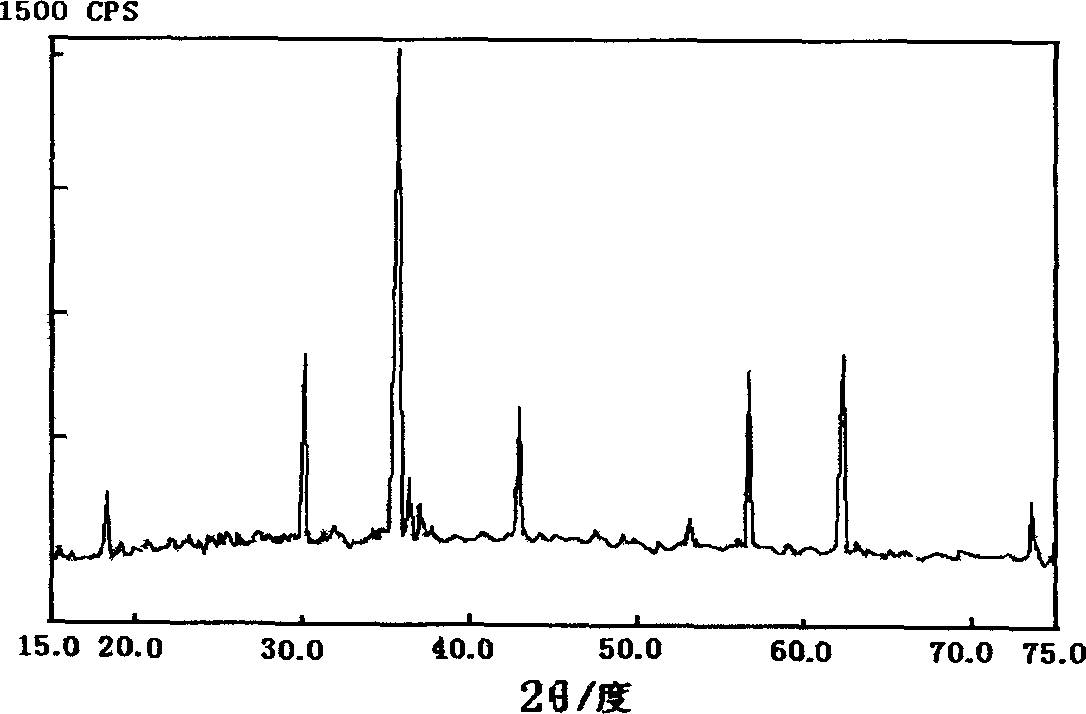
Method of prepn of ferrite from waste zinc-magnesium battery
InactiveCN1601661AAvoid pollutionRealize recyclingSolid waste disposalReclaiming serviceable partsManganesePhysical chemistry
The method includes following steps: (1) crashing zinc-manganese cells, solving coarsely separated iron-zinc-manganese so as to obtain solution of reaction predecessor: ferrous sulfate, zinc sulfate and manganese sulfate; (2) preparing predecessor of ferrite by reaction between mixed predecessors according to proportion and ammonium oxalate; (3) baking predecessor of ferrite in high temperature so as to obtain product of ferrite. The method carries out innoxiousness treatment and changes matter in cells to resources. Features are simple, economical and practical.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Electrolyte for magnesium battery
InactiveUS8722242B2Safe handlingReduce environmental impactAlkaline accumulatorsElectrode carriers/collectorsGrignard reagentMagnesium salt
Owner:TOYOTA JIDOSHA KK
Conversion reaction-based magnesium battery with high energy density
ActiveCN106532111AAvoid the problem of slow solid phase diffusion rateBroaden your optionsCell electrodesSecondary cellsHigh energySulfide
The invention relates to a conversion reaction-based magnesium battery with high energy density. A transition metal sulfide and / or a transition metal fluoride is used as a positive electrode material, a magnesium-lithium dual-salt solution is used as a non-aqueous electrolyte, and metal magnesium or magnesium alloy is used as a negative electrode. In the magnesium battery, the transition metal fluoride or the transition metal sulfide is used as the positive electrode material of such as magnesium-lithium hybrid battery system, the conversion reaction of the positive electrode material can be easily driven by lithium ions during the charge-discharge process, meanwhile, multi-electron conversion is achieved at a positive electrode and the negative electrode, the energy density of the positive electrode of the battery is improved to about 400 Wh / kg to the highest extent, and an energy density value can be comparable with that of a current lithium ion battery.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Preparation and application methods of nanometer carbon sphere-supported MXene composite material
InactiveCN109449402AImprove cycle stabilityIncrease spacingCell electrodesSecondary cellsSurface-active agentsMagnesium battery
The invention discloses preparation and application methods of a nanometer carbon sphere-supported MXene composite material. The preparation method comprises the steps of etching MAX-phase ceramic powder to obtain two-dimensional carbide MXene by a mixed solution of sodium fluoride and hydrochloric acid; synthesizing a nanometer carbon sphere by a hydrothermal method; and preparing a mixed solution from the MXene, a cationic surface active agent and the nanometer carbon sphere by electrostatic interaction, and performing stirring, centrifuging and drying under a room temperature, thereby obtaining the nanometer carbon sphere-supported MXene composite material powder. The preparation method is simple and safe and is low in cost. The nanometer carbon sphere-supported MXene composite materialis used as a magnesium battery positive electrode material, the interlayer distance of the MXene can be expanded, more surface active sites are exposed, ion transmission passages are increased, meanwhile, the problem of stack and agglomeration of an MXene piece layer during the circulation process is also solved, the magnesium storage capacity of the MXene is further substantially improved, the nanometer carbon sphere-supported MXene composite material has favorable cycle stability and is an excellent magnesium battery positive electrode material.
Owner:UNIV OF SCI & TECH BEIJING
Application method of titanium magnesium phosphate in anode material of chargeable magnesium battery
InactiveCN102931403AIncrease capacityIncrease discharge voltageCell electrodesMagnesium phosphateMagnesium orthophosphate
The invention discloses an application method of titanium magnesium phosphate in an anode material of a chargeable magnesium battery. The method comprises that the titanium magnesium phosphate with the chemical structural formula of Mg3Ti4(PO4)6 serves as a positive electrode, magnesium metal serves as a negative electrode, a Mg(AlCl2BuEt)2 / tetrahydrofuran or (PhMgCl)2-AlCl3 / tetrahydrofuran solution serves as an electrolyte solution, and the chargeable magnesium battery is formed. The magnesium battery produced by the application method has the advantages of being high in capacity, high in discharge voltage and good in rate performance.
Owner:SHANGHAI JIAO TONG UNIV
Rechargeable magnesium battery cathode material and preparation method thereof
ActiveCN107170968ANovel structureImprove performanceSecondary cellsPositive electrodesEnvironmental resistanceElectrical battery
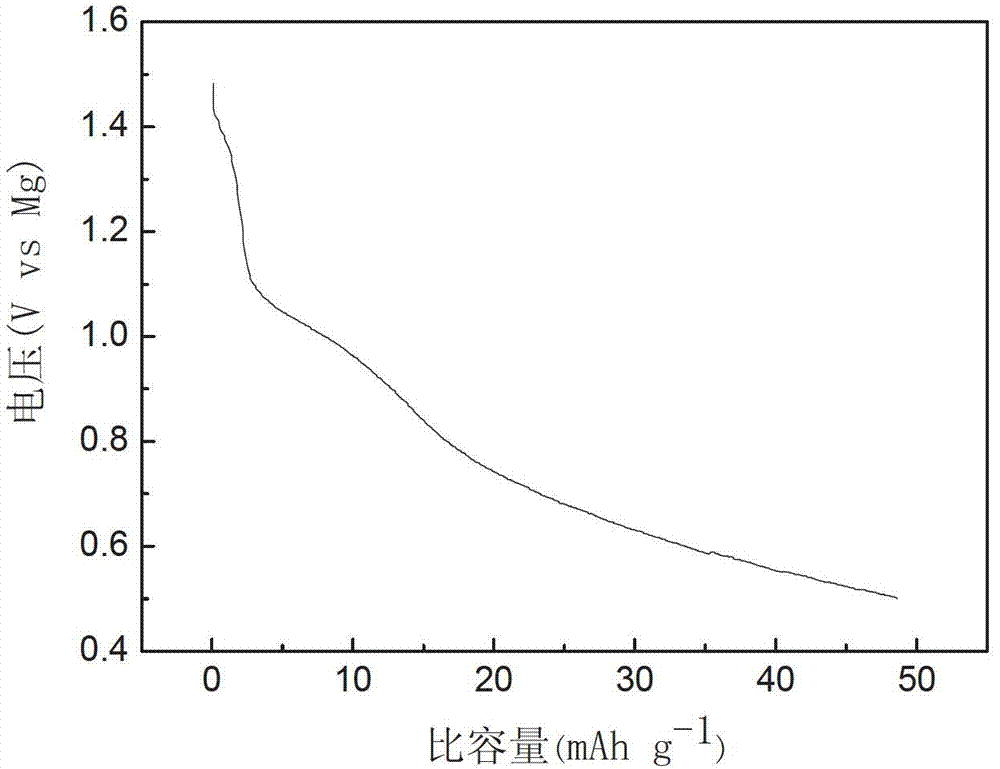
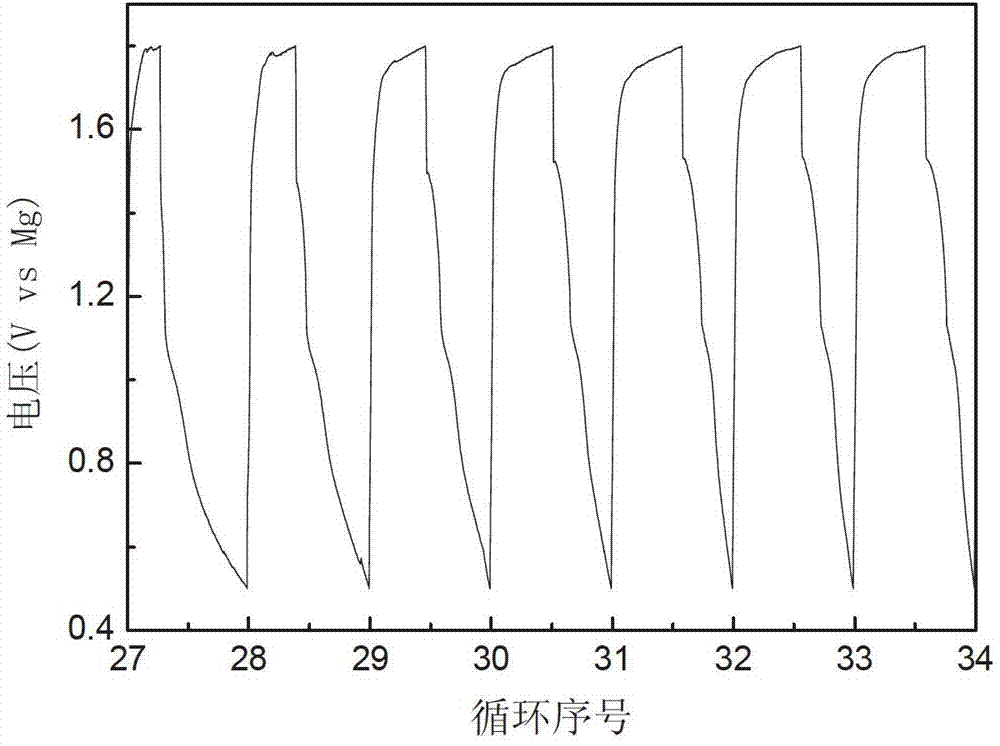
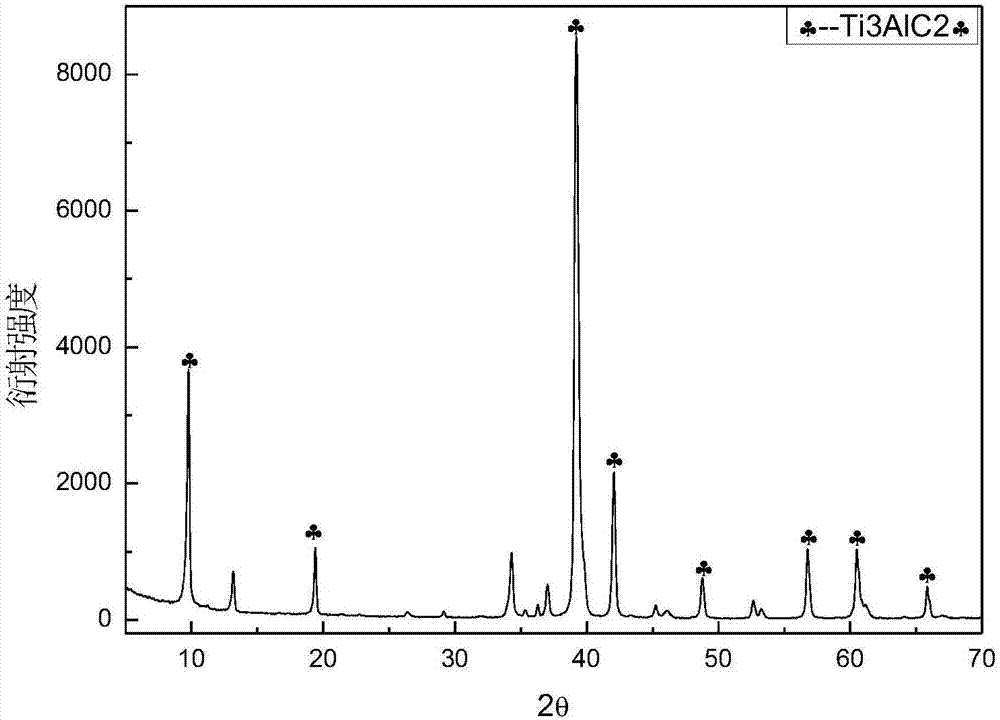
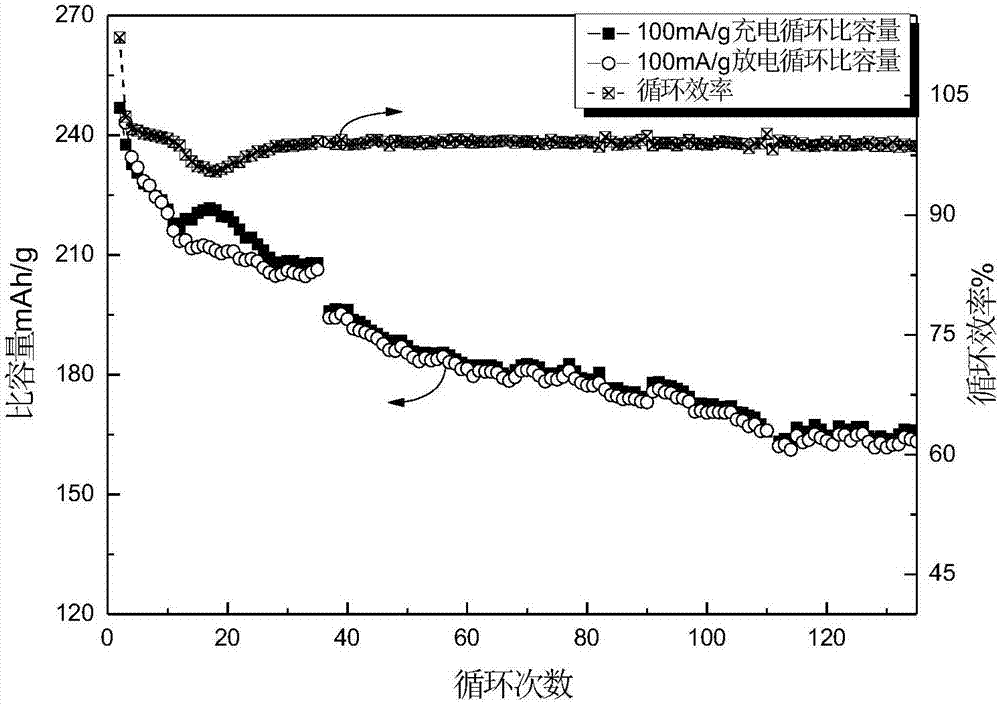
The invention relates to a rechargeable magnesium battery cathode material. The percentages by weight of the chemical components of the rechargeable magnesium battery cathode material are: 80 to 90 percent of titanium dioxide, 5 to 10 percent of activated carbon and 5 to 10 percent of carbon nanotubes. A preparation method for the rechargeable magnesium battery cathode material mainly includes the following steps: the powder of three types of elements, i.e. titanium, aluminium and carbon, is sintered by spark plasma, so that Ti3AlC2 is synthesized; Ti3C2Tx is obtained by hydrofluoric acid erosion; Ti3C2Tx is put into glucose or sucrose solution, and activated carbon-coated Ti3C2Tx is obtained by hydrothermal carbonization; aqueous hydrogen peroxide solution is added into activated carbon-coated Ti3C2Tx, and after oxidation in a hydrothermal reaction kettle, activated carbon-coated two-dimensional layered titanium dioxide is obtained; the activated carbon-coated two-dimensional layered titanium dioxide is mixed with the carbon nanotubes, an appropriated amount of deionized water is added, and after stirring and ultrasonic oscillation, the rechargeable magnesium battery cathode material is obtained. The rechargeable magnesium battery cathode material obtained by the invention is nontoxic, harmless, safe and environment-friendly, the structure is novel, and both the cycle performance and rate capability of the magnesium battery are greatly enhanced.
Owner:YANSHAN UNIV
Non-aqueous electrolyte for high voltage rechargeable magnesium batteries
InactiveUS20140099557A1Improve conductivityEasy to depositOrganic electrolyte cellsSecondary cellsGrignard reagentMetallic materials
An electrolyte for use in electrochemical cells is provided. The properties of the electrolyte include high conductivity, high Coulombic efficiency, and an electrochemical window that can exceed 3.5 V vs. Mg / Mg+2. The use of the electrolyte promotes the electrochemical deposition and dissolution of Mg without the use of any Grignard reagents, other organometallic materials, tetraphenyl borate, or tetrachloroaluminate derived anions. Other Mg-containing electrolyte systems that are expected to be suitable for use in secondary batteries are also described.
Owner:PELLION TECH
Magnesium borohydride and its derivatives as magnesium ion transfer media
InactiveUS20140038037A1Monoborane/diborane hydridesOrganic electrolyte cellsMagnesium saltMolten salt
An electrolyte for a magnesium battery includes a magnesium salt having the formula MgBaHbXy where a=2-12, b=0-12 y=0-8 wherein when b=0 X is O-alkyl and when b=1-11 X is O-alkyl or F. The electrolyte also includes a solvent, the magnesium salt being dissolved in the solvent. Various solvents including aprotic solvents and molten salts such as ionic liquids may be utilized.
Owner:TOYOTA MOTOR ENGINEERING & MANUFACTURING NORTH AMERICA
Rechargeable magnesium battery
InactiveCN104538669AImprove discharge capacityAbundant raw materialsElectrolyte accumulators manufactureMetalTitanium dioxide
The invention discloses a rechargeable magnesium battery. An anode material is titanium dioxide or element-doped titanium dioxide, a cathode material is metal magnesium or a magnesium alloy, and electrolyte is borohydride magnesium dissolved in organic ether. The invention also provides a preparation method of the rechargeable magnesium battery. The rechargeable magnesium battery has advantages of high discharging capacity and stability in circulation and is very good in application prospect as a green energy resource.
Owner:SHANGHAI JIAO TONG UNIV
Preparation method of vanadium dioxide nano material and application in rechargeable magnesium battery
ActiveCN106904653AGood electrochemical performance for magnesium storageImprove electrochemical performanceMaterial nanotechnologyCell electrodesMagnesium batteryRaw material
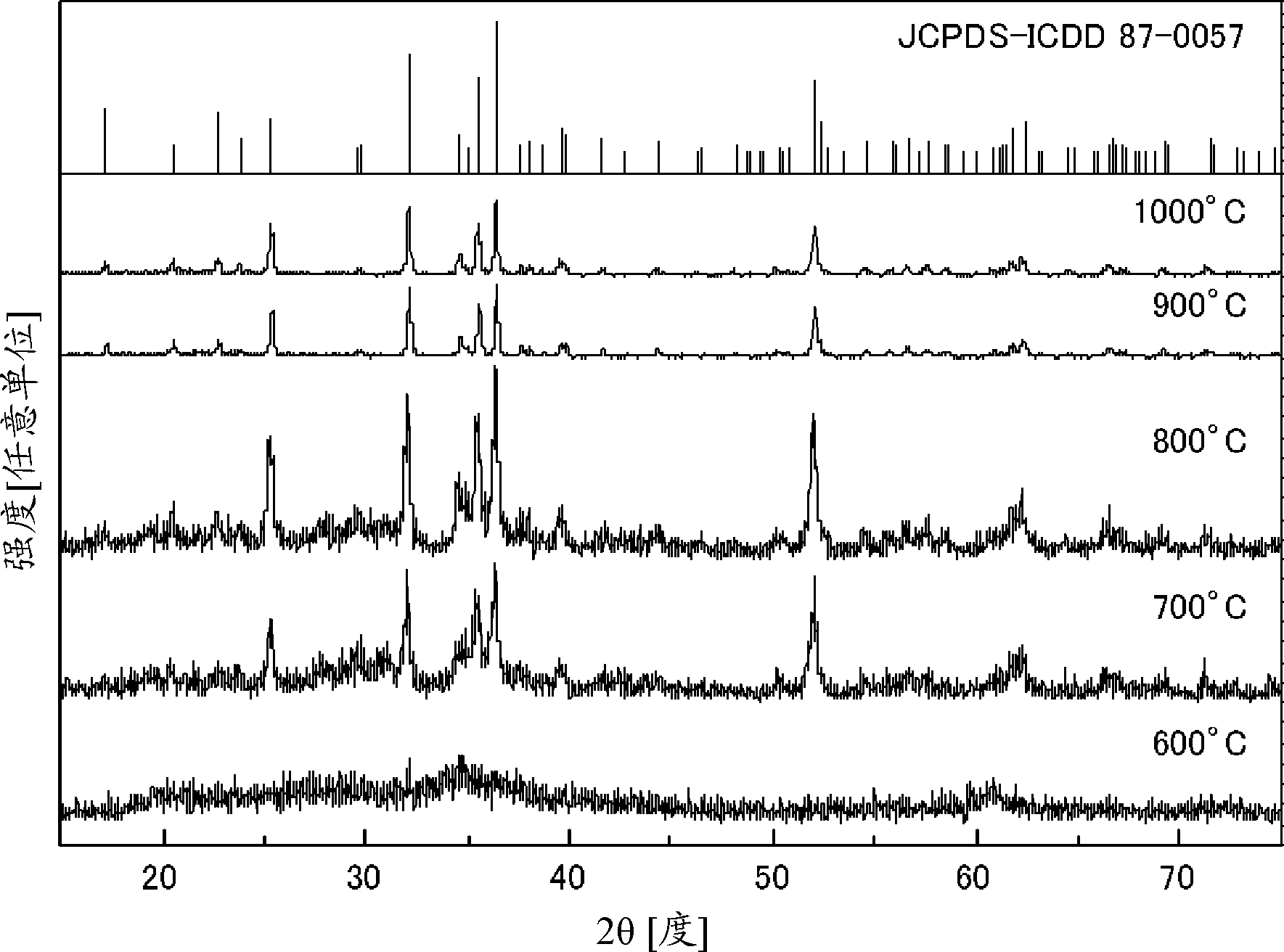
The invention relates to a preparation method of a vanadium dioxide nano material and application in a rechargeable magnesium battery. The vanadium dioxide nano material, of which the crystal structure is a phase B, is prepared by taking vanadium pentoxide, citric acid and a surfactant which are low in cost as raw materials and adopting a one-pot hydrothermal method; the vanadium dioxide nano material is high in purity and good in crystallization performance, and the preparation method is easy to control. When the phase-B vanadium dioxide nano material is used as a cathode material of the rechargeable magnesium battery, at the current density of 50 mA / g, the first specific discharge capacity can be up to 394.2 mAh / g; after the battery is cycled for 60 circles, the specific discharge capacity still can be kept at 205.9 mAh / g, and a discharge voltage platform reaches 2.1 V (vs.Mg<2+> / Mg), so that relatively high electrochemical magnesium removal and embedding performance is achieved.
Owner:上海鑫忆丹新材料有限公司
Carboranyl magnesium electrolyte for magnesium battery
An electrochemical device is provided having a carboranyl magnesium electrolyte. Specifically the disclosure relates to an electrochemical device having a magnesium anode, a cathode, and a current collector made of non-noble metal, and a carboranyl magnesium electrolyte. In contact with the electrolyte, the non-noble metal cathode current collector has unusually high oxidative stability >3.0V vs. a magnesium reference. Processes for making the electrochemical device are additionally provided.
Owner:TOYOTA JIDOSHA KK
Rechargeable magnesium ion cell components and assembly
InactiveUS8361661B2Cost-effective fabricationHigh voltageSilver accumulatorsElectrode carriers/collectorsEngineeringMagnesium ion
A magnesium battery electrode assembly is described, including a current collector comprising a carbonaceous material and an electrode layer comprising an electrode active material disposed on the current collector.
Owner:PELLION TECH
Electrolyte solution and magnesium battery including the same
An electrolytic solution including: a magnesium salt; a non-aqueous organic solvent; and an anion receptor, wherein the anion receptor comprises at least one compound selected from the group consisting of compounds represented by Formulae 1 and 2 below:where A, m, p1, P2, P3, q1, RA, Ra, R1 through R6, and Ry are the same as described in the detailed description section.
Owner:SAMSUNG ELECTRONICS CO LTD
Inorganic magnesium solid electrolyte, magnesium battery, and method for producing inorganic magnesium solid electrolyte
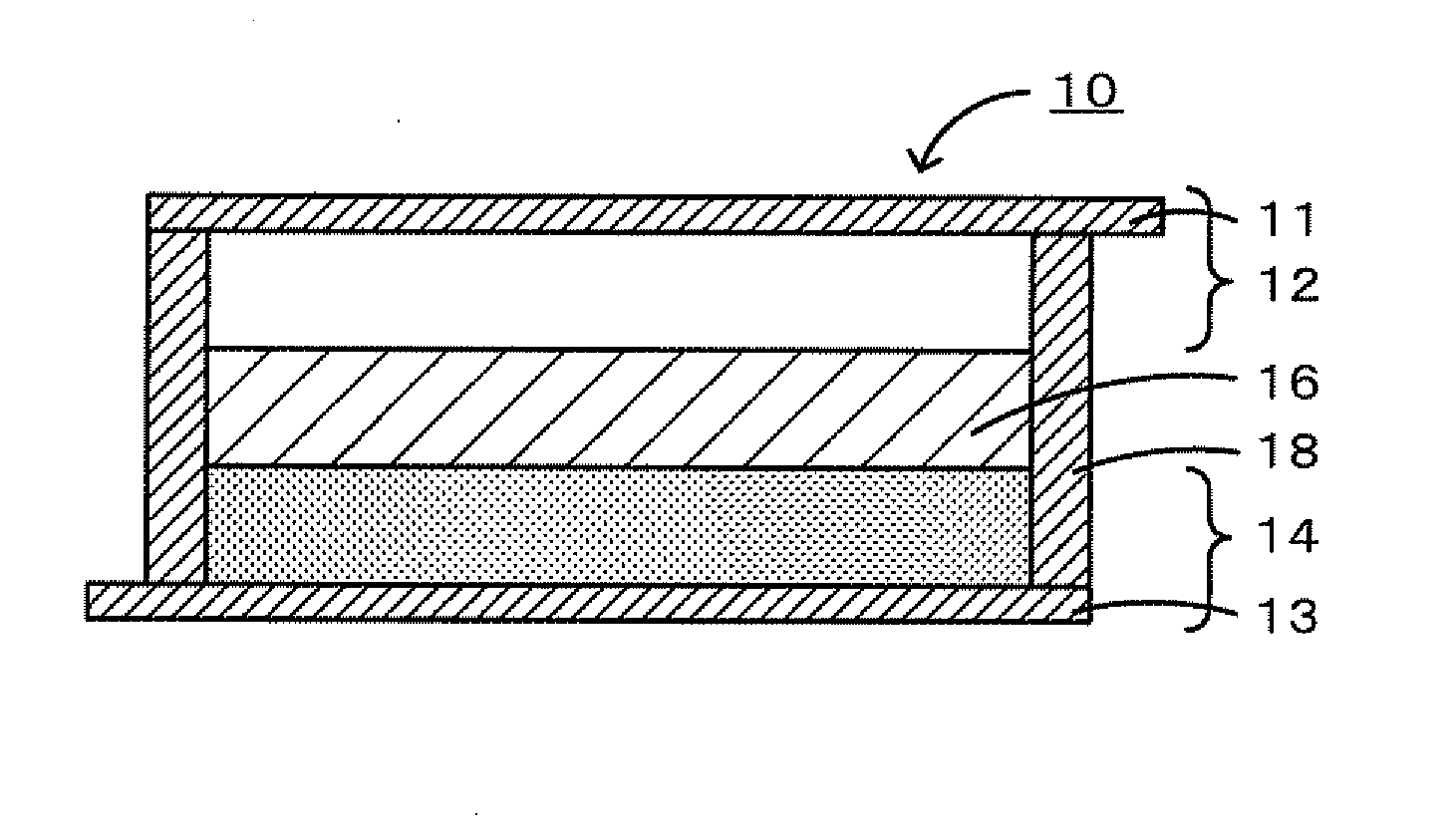
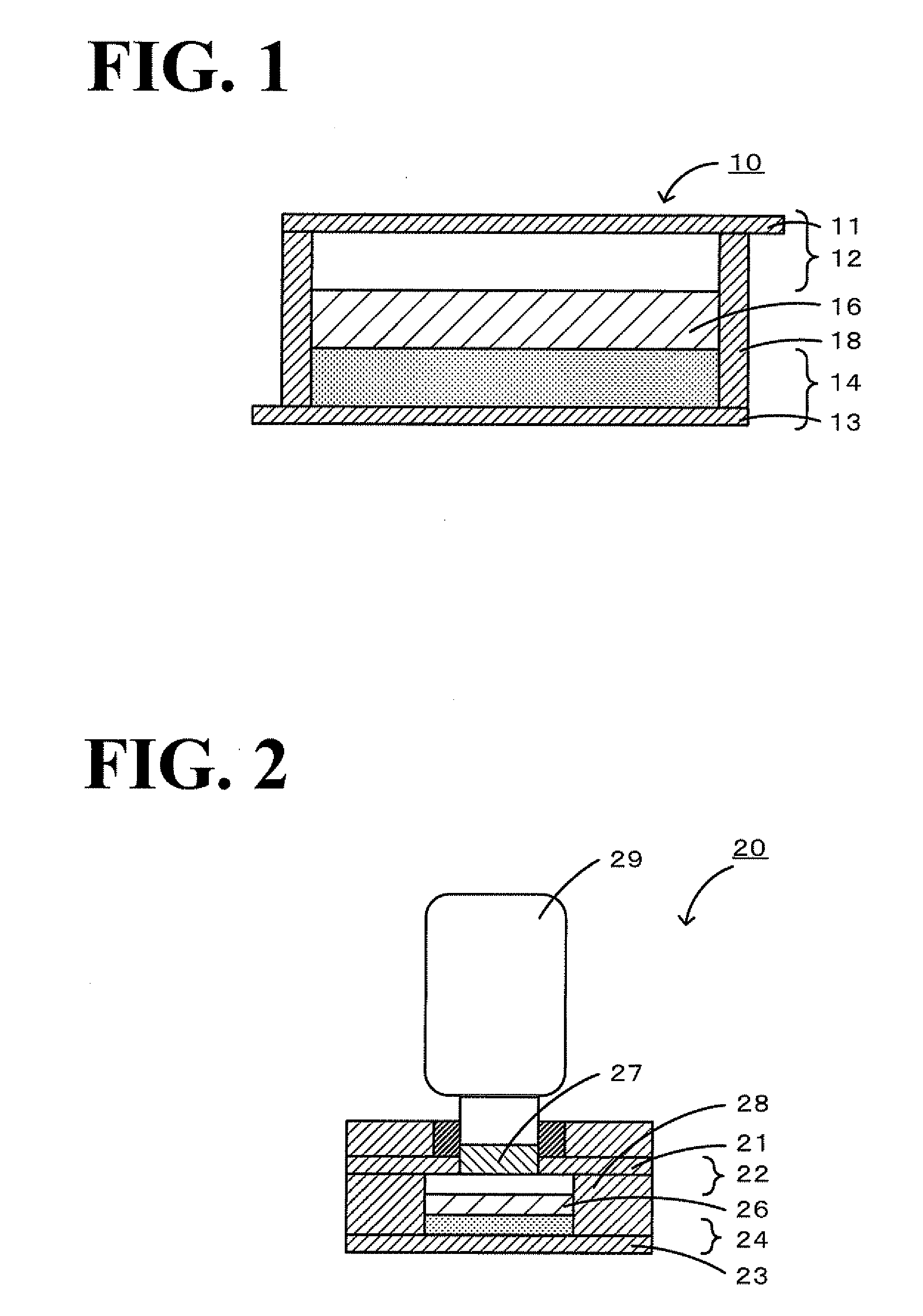
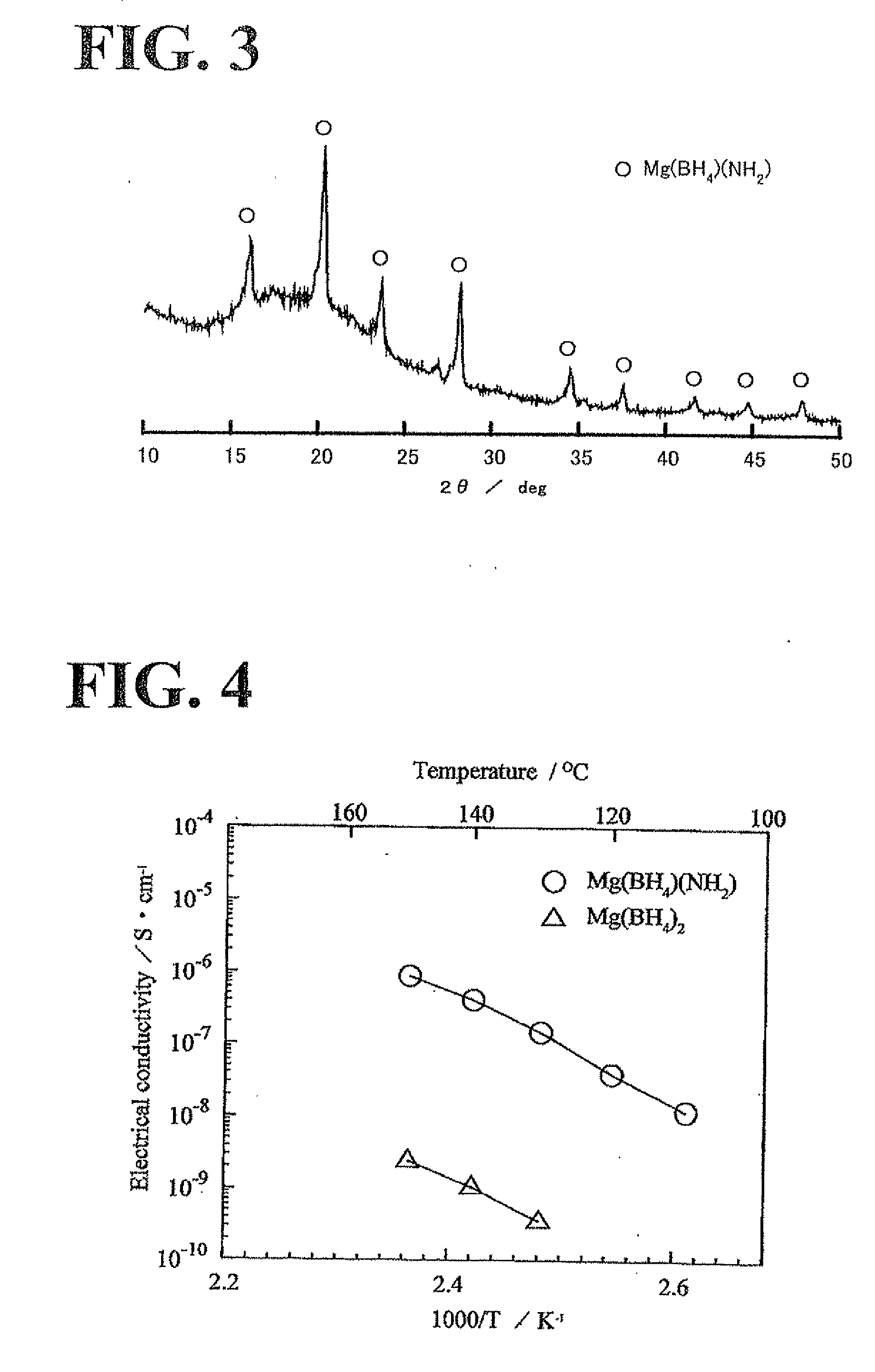
A magnesium battery 10 according to the present invention includes a positive electrode 12, a negative electrode 14 having a magnesium-containing negative electrode active material, and an inorganic magnesium solid electrolyte 16 that is interposed between the positive electrode 12 and the negative electrode 14, has a complex ion structure that contains magnesium and hydrogen, and conducts magnesium ions. The inorganic magnesium solid electrolyte 16 may contain a compound having at least one selected from boron and nitrogen. The inorganic magnesium solid electrolyte may be produced by a production method that includes a heat-treatment step of mixing and heating Mg(BH4)2 and Mg(NH2)2 to form a compound having a complex ion structure that contains magnesium and hydrogen.
Owner:TOYOTA CENT RES & DEV LAB INC
Cathodes and electrolytes for rechargeable magnesium batteries and methods of manufacture
ActiveUS20150010832A1Silver accumulatorsOrganic electrolyte cellsRechargeable cellElectrochemical cell
The invention relates to Chevrel-phase materials and methods of preparing these materials utilizing a precursor approach. The Chevrel-phase materials are useful in assembling electrodes, e.g., cathodes, for use in electrochemical cells, such as rechargeable batteries. The Chevrel-phase materials have a general formula of Mo6Z8 and the precursors have a general formula of MxMo6Z8. The cathode containing the Chevrel-phase material in accordance with the invention can be combined with a magnesium-containing anode and an electrolyte.
Owner:UNIVERSITY OF PITTSBURGH +1
Application method of taking nano transition metal sulfide as positive electrode material of rechargeable magnesium battery
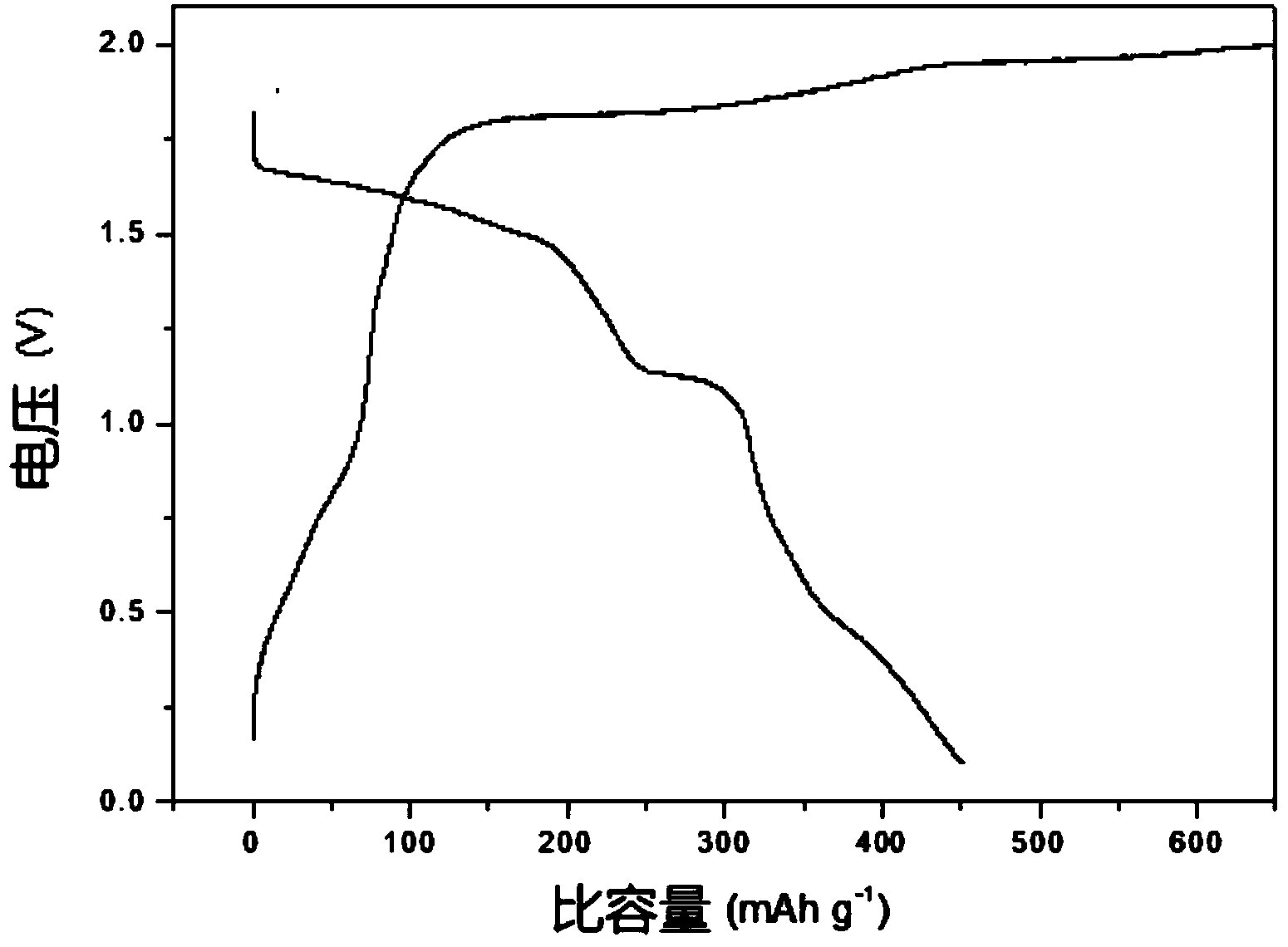
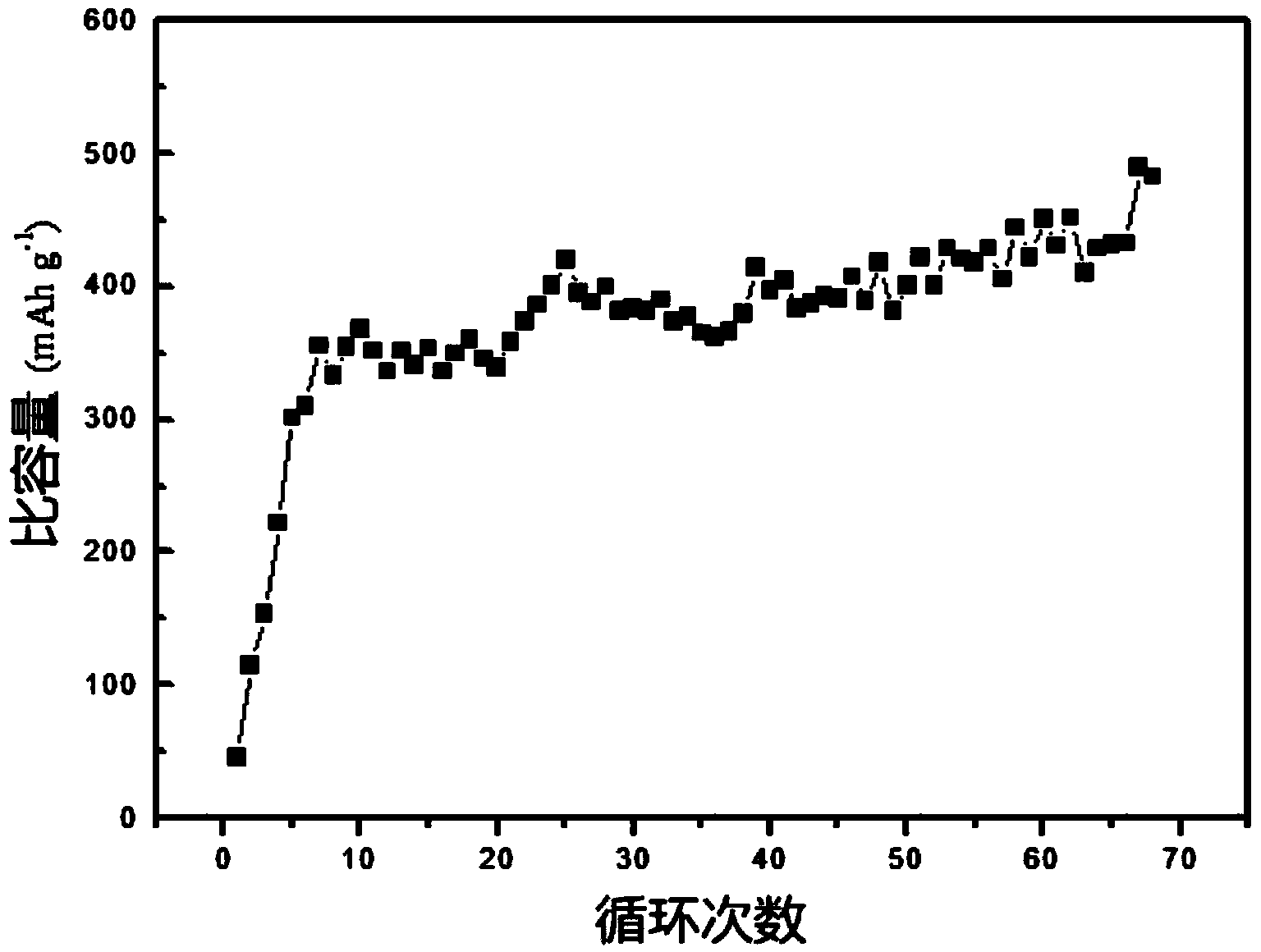
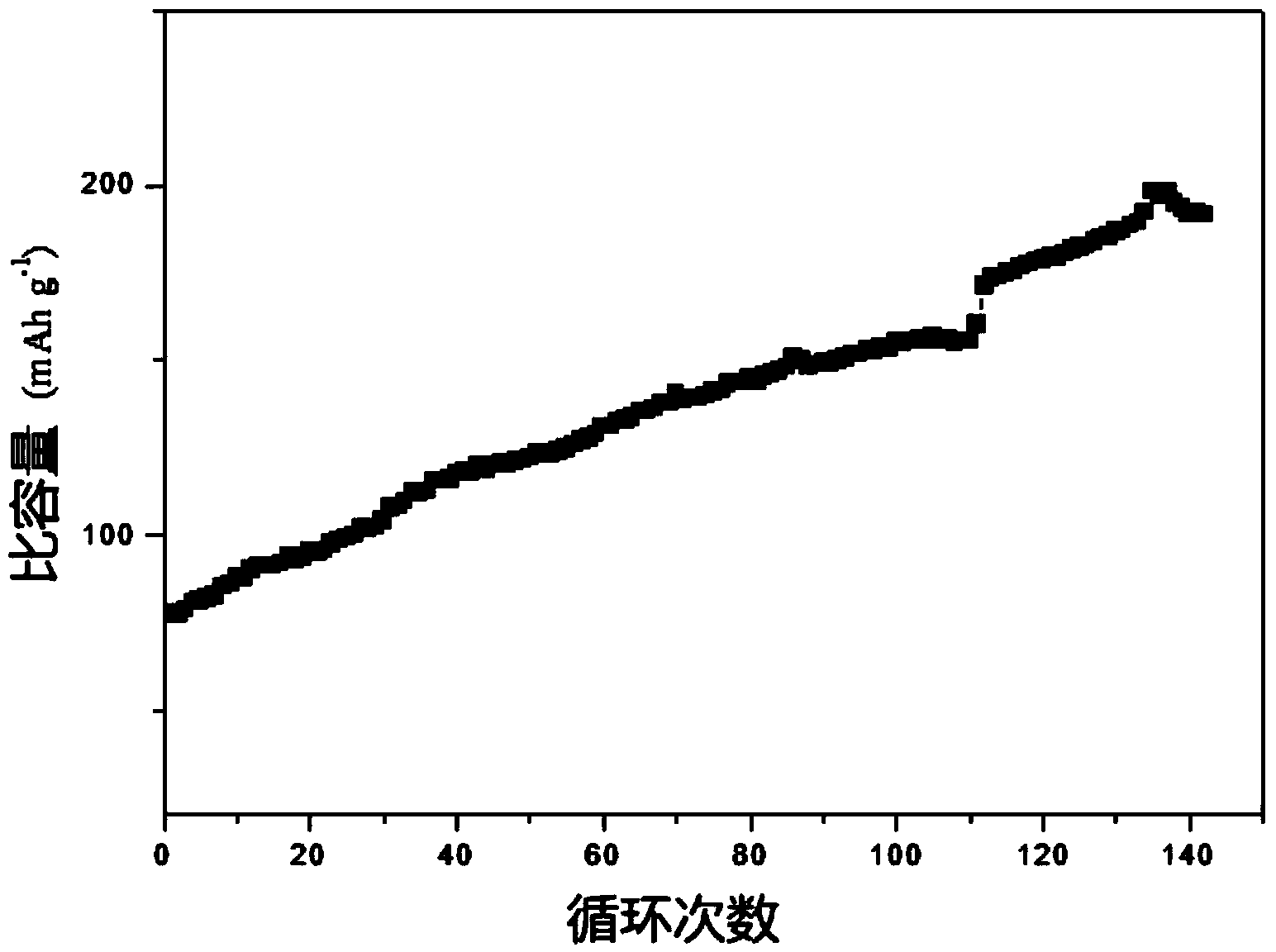
ActiveCN103872321AImprove electrochemical performanceStable charging and discharging platformSecondary cellsPositive electrodesMetallic sulfideMagnesium ion
The invention discloses an application method of taking nano transition metal sulfide as a positive electrode material of a rechargeable magnesium battery. The application method comprises the following steps: synthesizing a transition metal salt and a sulfur source compound to form a nano transition metal sulfide by a hydrothermal method under reaction conditions that the temperature is 160-220 DEG C and the heat is kept for 12-24 hours; by taking the nano transition metal sulfide as an active substance, adding a conductive agent and a binding agent to prepare a positive plate containing the nano transition metal sulfide; and by taking the positive plate as a positive electrode and pure magnesium metal as a negative electrode, preparing the rechargeable magnesium battery. The nano transition metal sulfide material is used as the active substance of the rechargeable magnesium battery and reversible embedding of divalent magnesium ions can be carried out, so that the rechargeable magnesium battery prepared by the method has a stable charging / discharging platform and the specific discharge capacity is higher than 400mAh / g under the current density of 20mA / g; the rechargeable magnesium battery has no capacity fading after being circulated for 70 times.
Owner:SHANGHAI JIAO TONG UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com