Rechargeable magnesium battery cathode material and preparation method thereof
A cathode material, magnesium battery technology, applied in secondary batteries, battery electrodes, positive electrodes, etc., can solve the problems of complicated preparation process, low battery efficiency, low theoretical capacity, etc., achieve broad application prospects, enhance conductivity, Excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
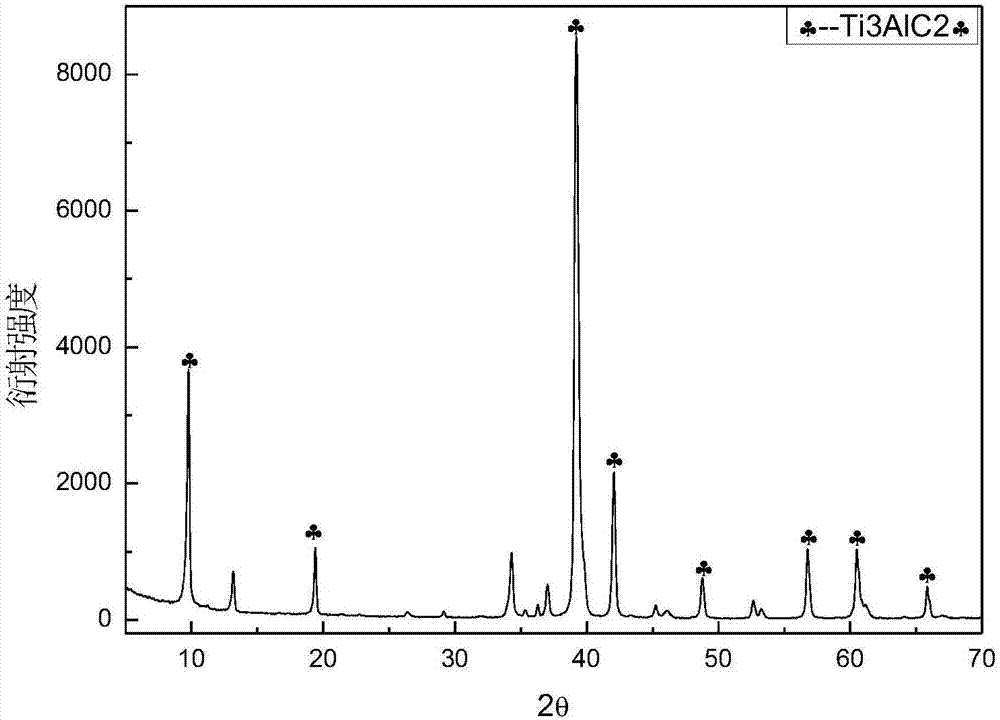
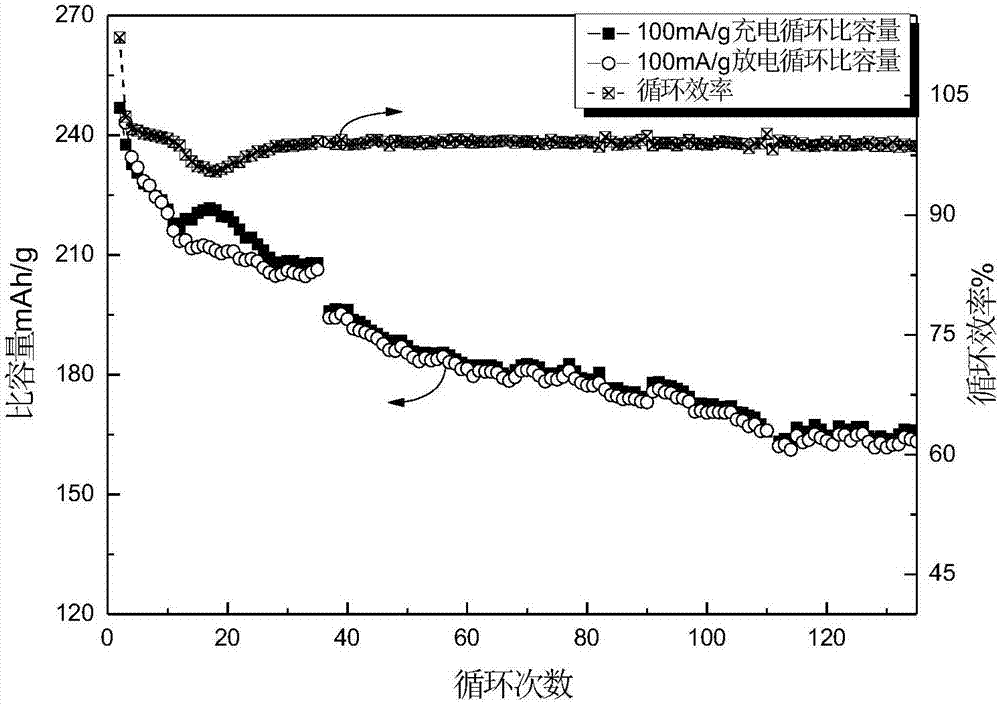
[0029] Use the elemental powder of titanium, aluminum and carbon as raw materials, mix according to the ratio of Ti:Al:C=3:1.1:1.9, add grinding balls at a ball-to-material ratio of 10:1, and operate at 300r / min under argon atmosphere After ball milling for 12 hours, the powder was taken out and sintered by SPS (Spark Plasma Sintering) at a pressure of 50 MPa and a heating rate of 100 °C / min to 1350 °C, kept for 10 min, and argon assisted cooling to room temperature. The XRD pattern of the obtained product is as follows figure 1 Shown, indicating that the obtained product is Ti 3 AlC 2 . Ti 3 AlC 2 The surface is polished clean, ground into powder, and the powder below 200 mesh is sieved, soaked in 40% hydrofluoric acid at 40°C for 12 hours, washed with deionized water until neutral, centrifuged, and dried to obtain Ti 3 C 2 T x (T is -F, -OH or -O-); take 0.5g Ti 3 C 2 T x Added to 20mL of 1% glucose solution, ultrasonically oscillated for 30min, transferred to a hyd...
Embodiment 2
[0032] Use the elemental powder of titanium, aluminum and carbon as raw materials, mix according to the ratio of Ti:Al:C=3:1.1:1.9, add grinding balls at a ball-to-material ratio of 10:1, and operate at 300r / min under argon atmosphere After ball milling for 12 hours, the powder was taken out and sintered by SPS (Spark Plasma Sintering) at a pressure of 50 MPa and a heating rate of 100 °C / min to 1350 °C, kept for 10 min, and cooled to room temperature with argon gas assistance. The obtained product Ti 3 AlC 2 ; 3 AlC 2 The surface is polished clean, ground into powder, and the powder below 200 mesh is sieved, soaked in 40% hydrofluoric acid at 40°C for 12 hours, washed with deionized water until neutral, centrifuged, and dried to obtain Ti 3 C 2 T x (T is -F, -OH or -O-); take 0.2g Ti 3 C 2 T x Add it into 50mL of 0.5% glucose solution, ultrasonically oscillate for 30min, transfer to a hydrothermal reactor, react at 220°C for 12h, cool to room temperature, wash and dry t...
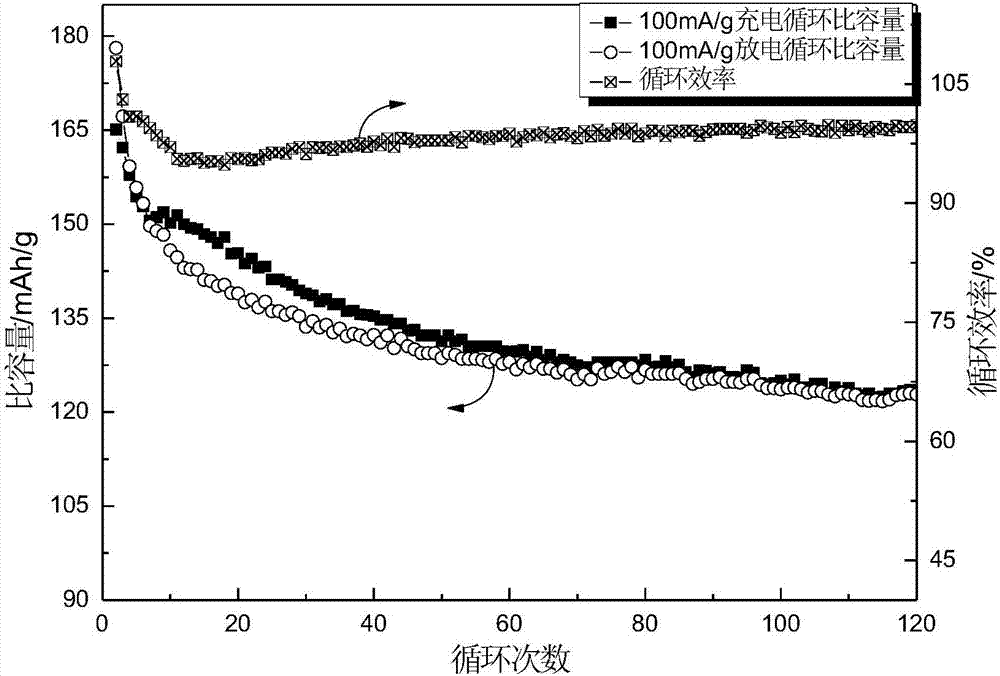
Embodiment 3
[0035] Use the elemental powder of titanium, aluminum and carbon as the raw material, mix it according to the ratio of Ti:Al:C=3:1.1:1.9, put it into the grinding ball with the ratio of ball to material 10:1, 300r / min under argon atmosphere After ball milling for 12 hours, the powder was taken out and sintered by SPS (Spark Plasma Sintering) at a pressure of 50 MPa and a heating rate of 100°C / min to 1350°C, held for 10 minutes, and argon assisted cooling to room temperature. The resulting product was Ti 3 AlC 2 ; 3 AlC 2 The surface is polished clean, ground into powder, and the powder below 200 mesh is sieved, soaked in 40% hydrofluoric acid at 40°C for 12 hours, washed with deionized water until neutral, centrifuged, and dried to obtain Ti 3 C 2 T x (T is -F, -OH or -O-); take 2g Ti 3 C 2 T x Add it into 50mL of 2% glucose (or sucrose) solution, ultrasonically shake for 30min, then transfer to a hydrothermal reaction kettle, react at 220°C for 12h, cool to room temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com