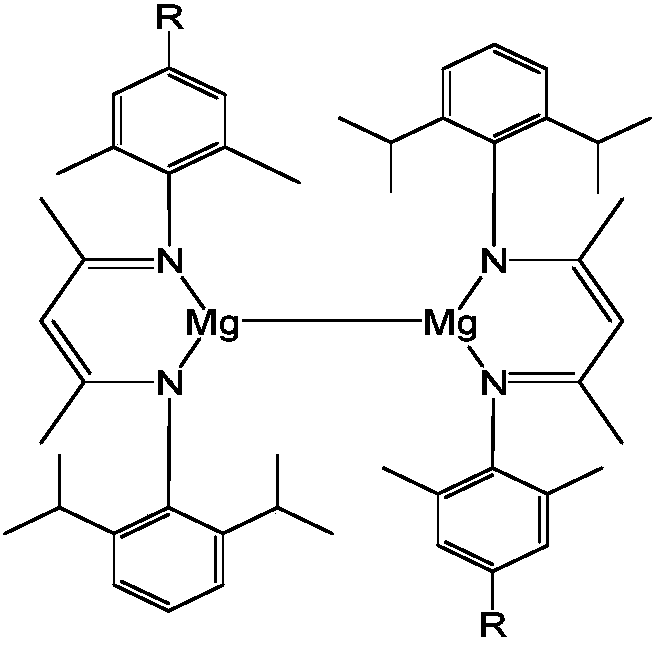
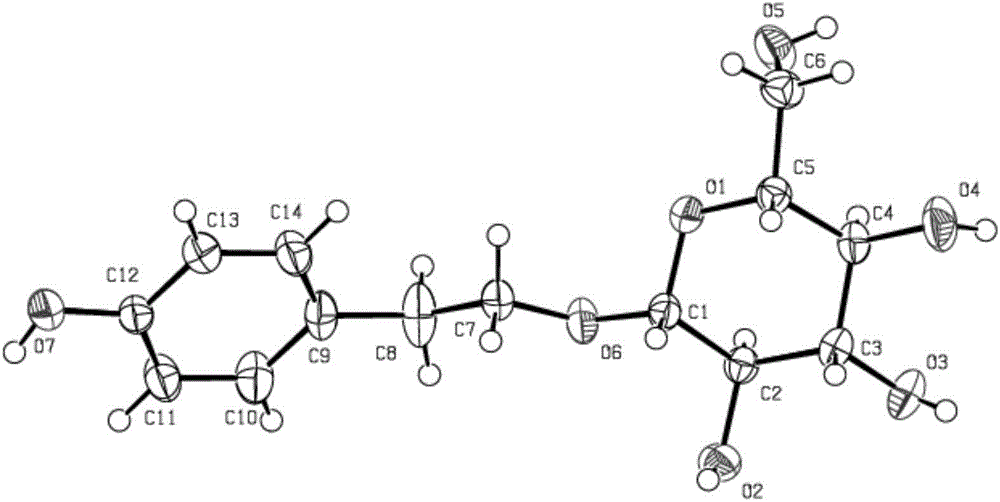
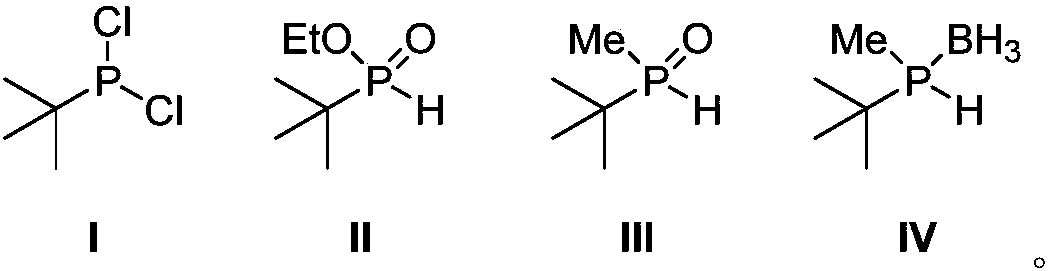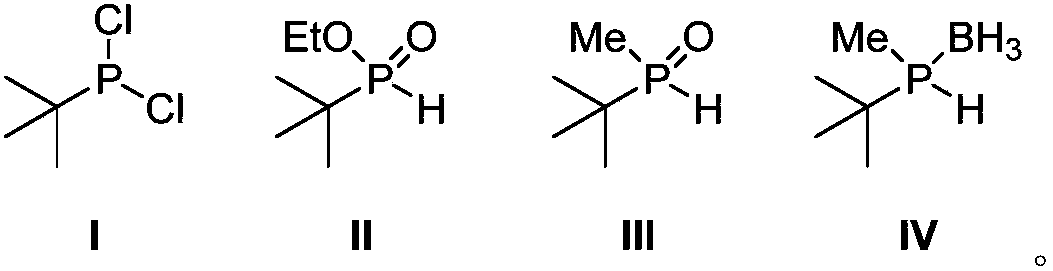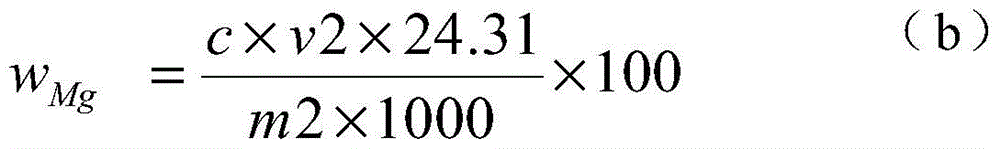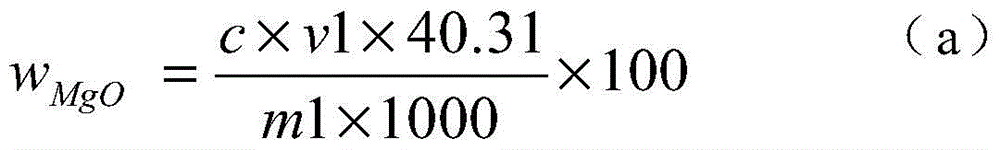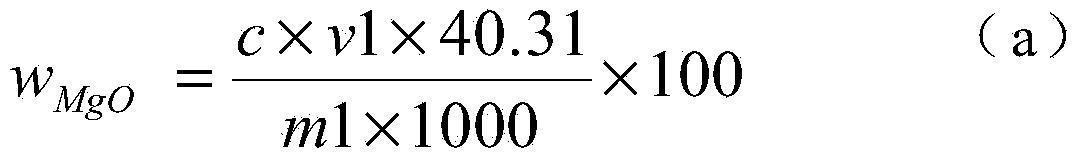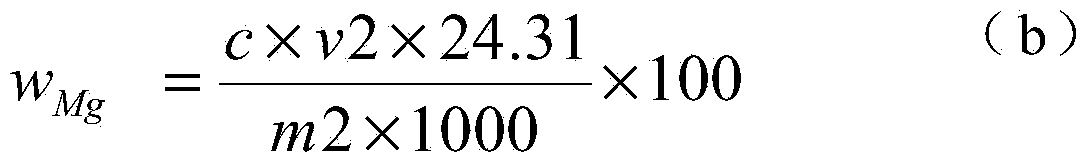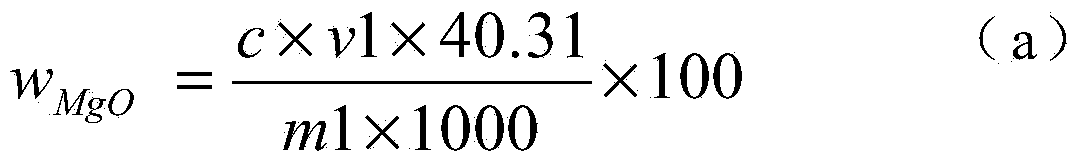Patents
Literature
32 results about "Magnesium iodide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
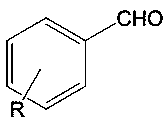
Magnesium iodide is the name for the chemical compounds with the formulas MgI₂ and its various hydrates MgI₂(H₂O)ₓ. These salts are typical ionic halides, being highly soluble in water.
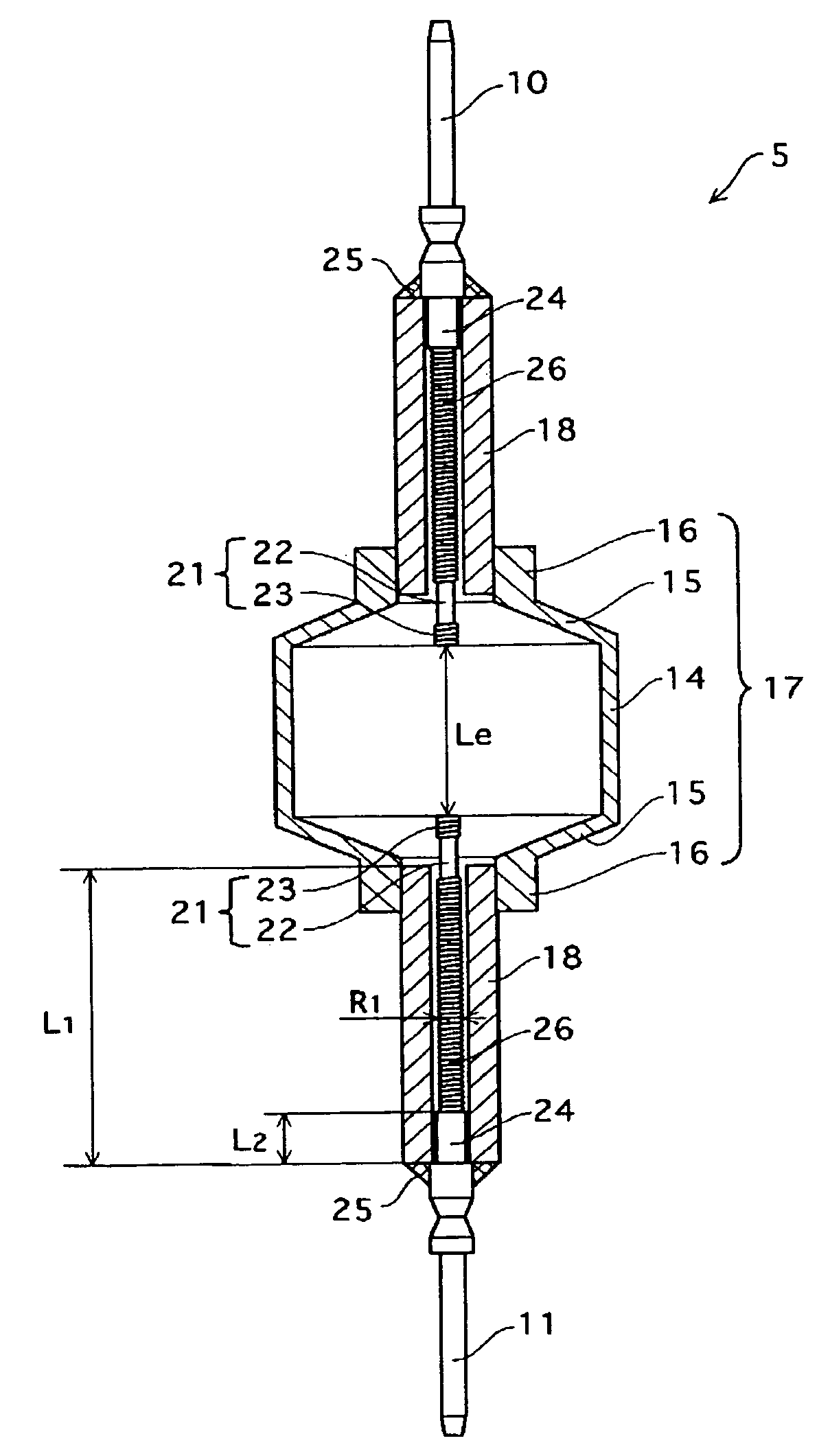
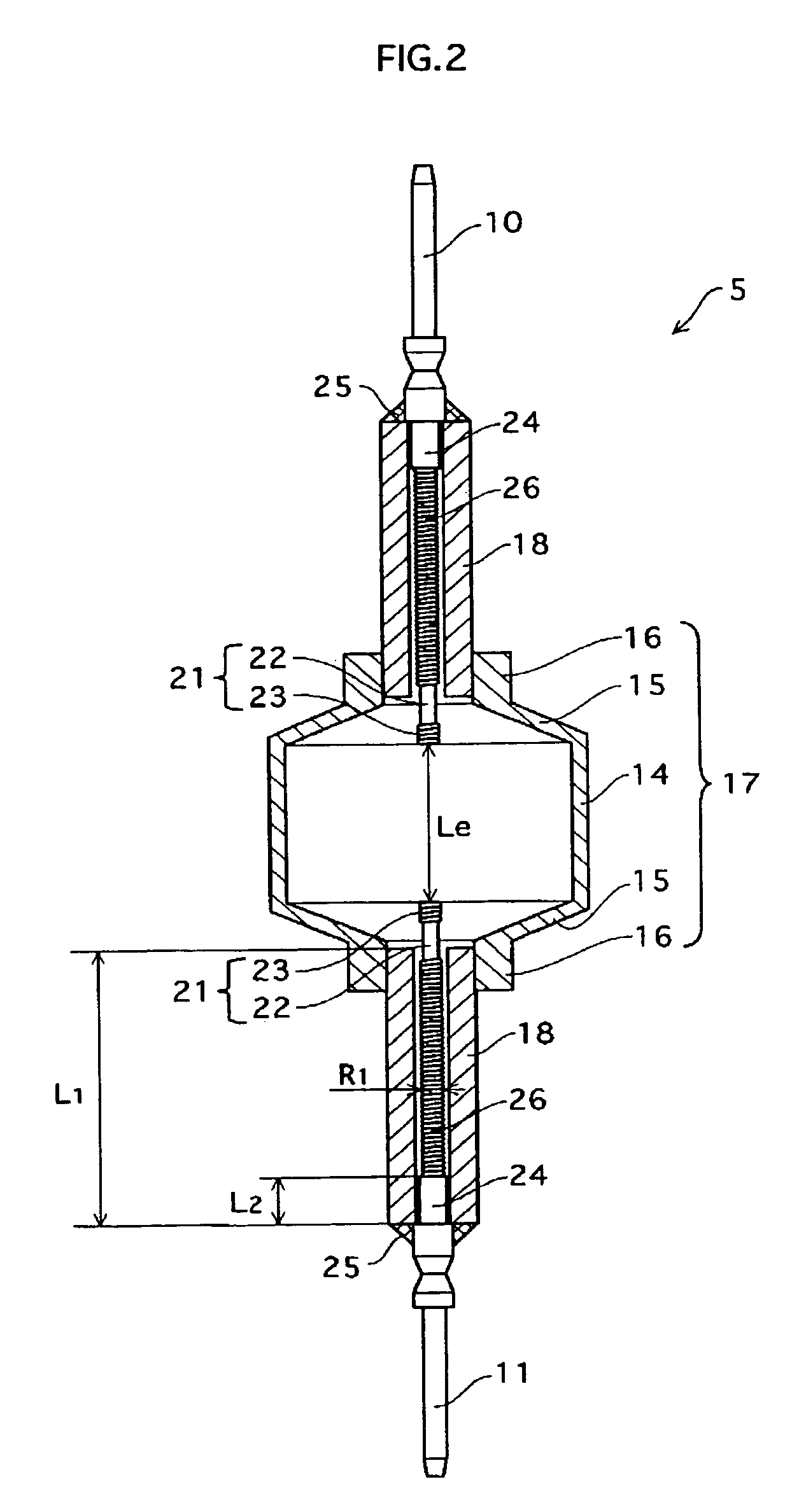
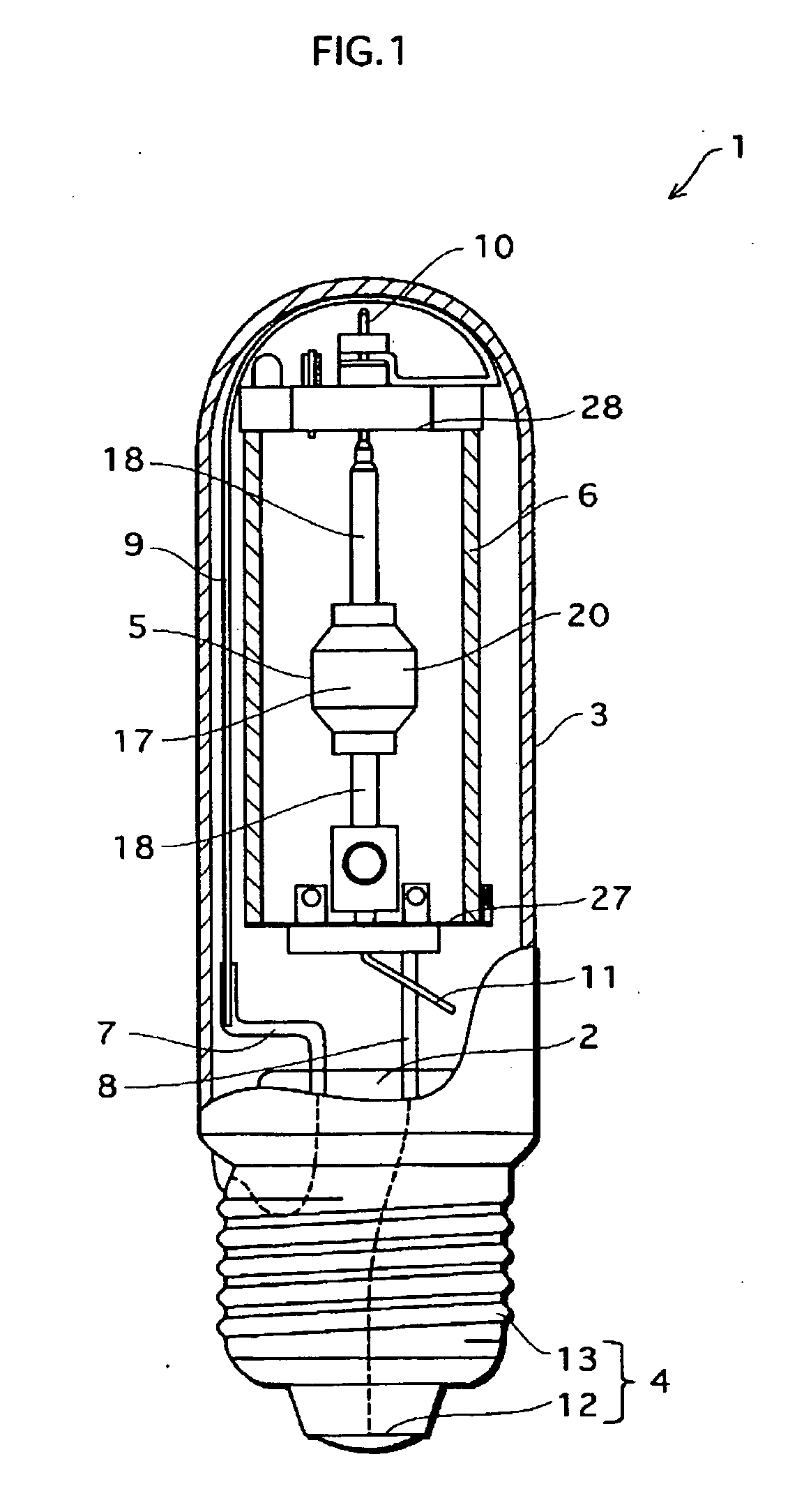
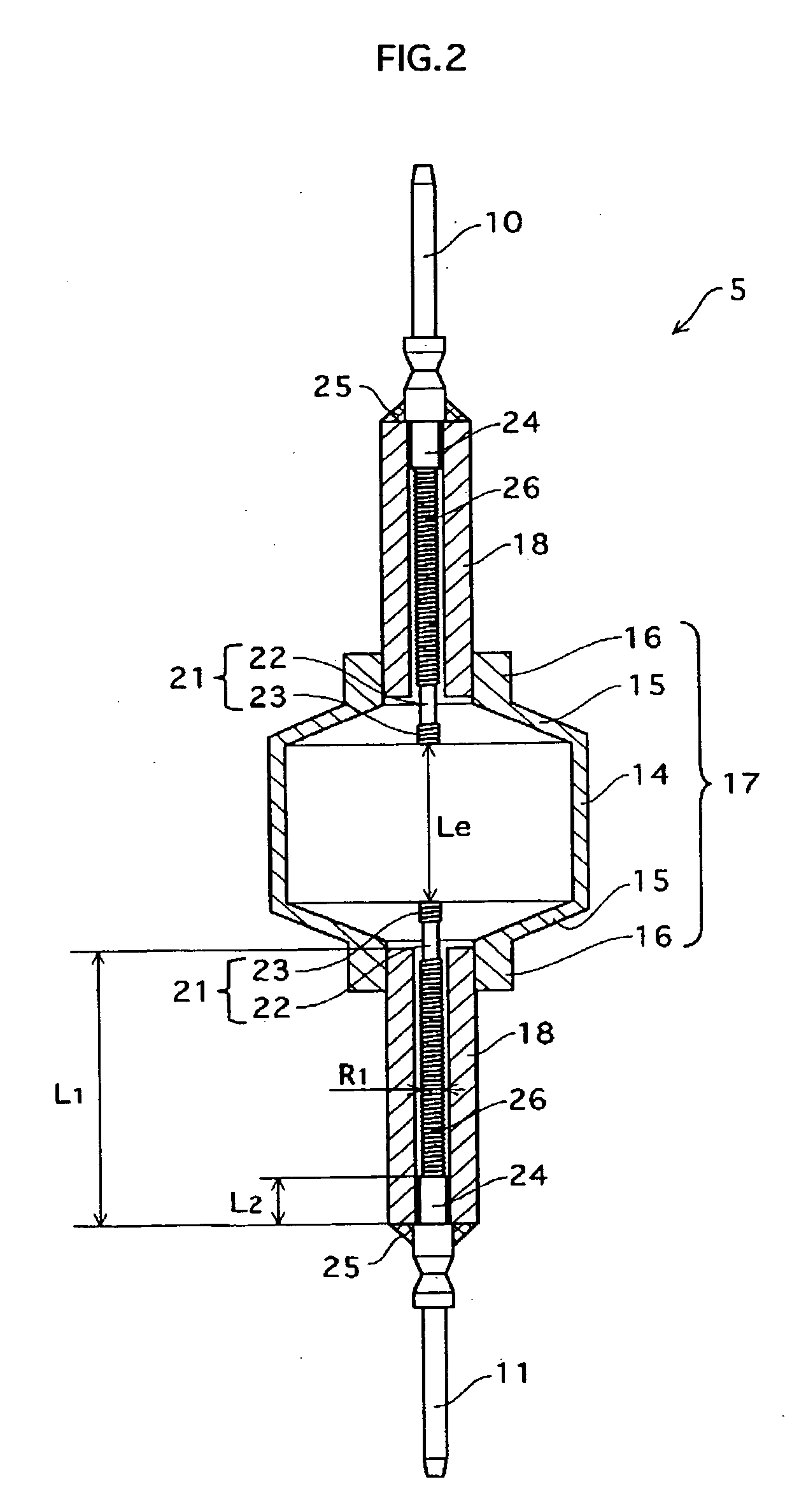
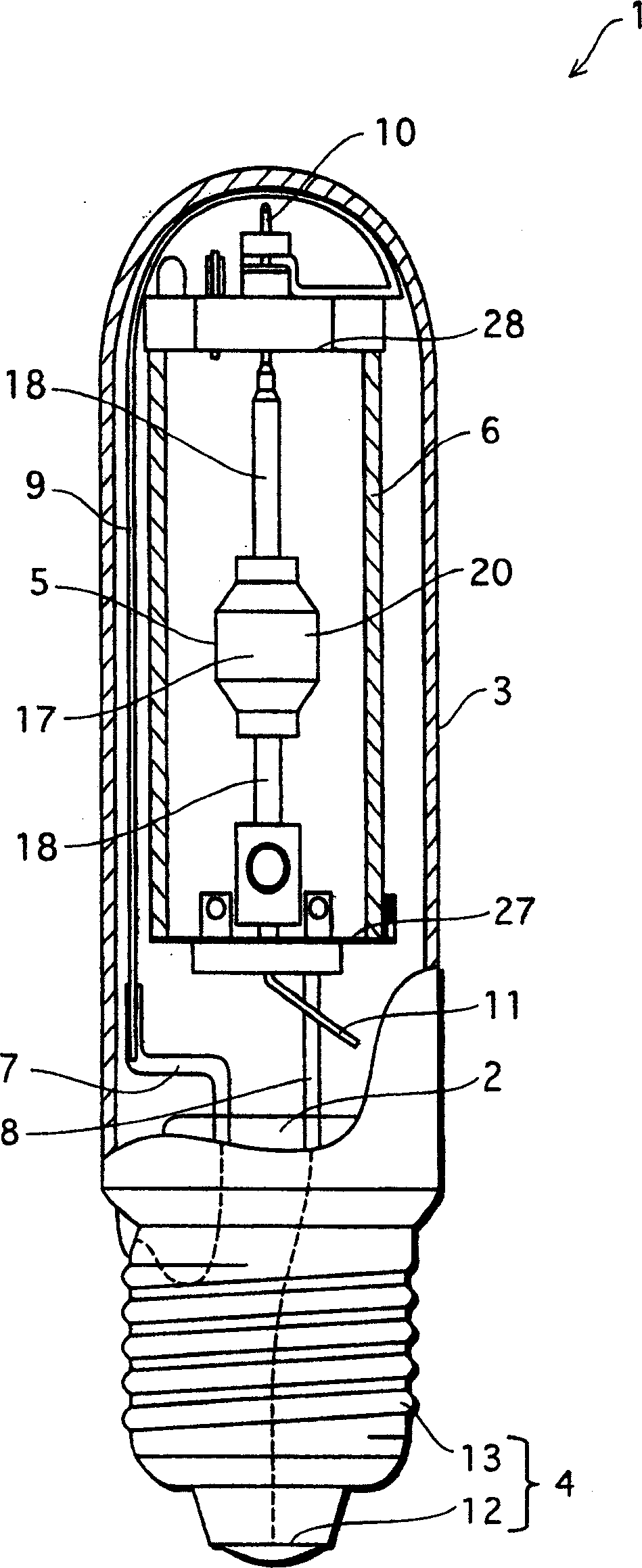
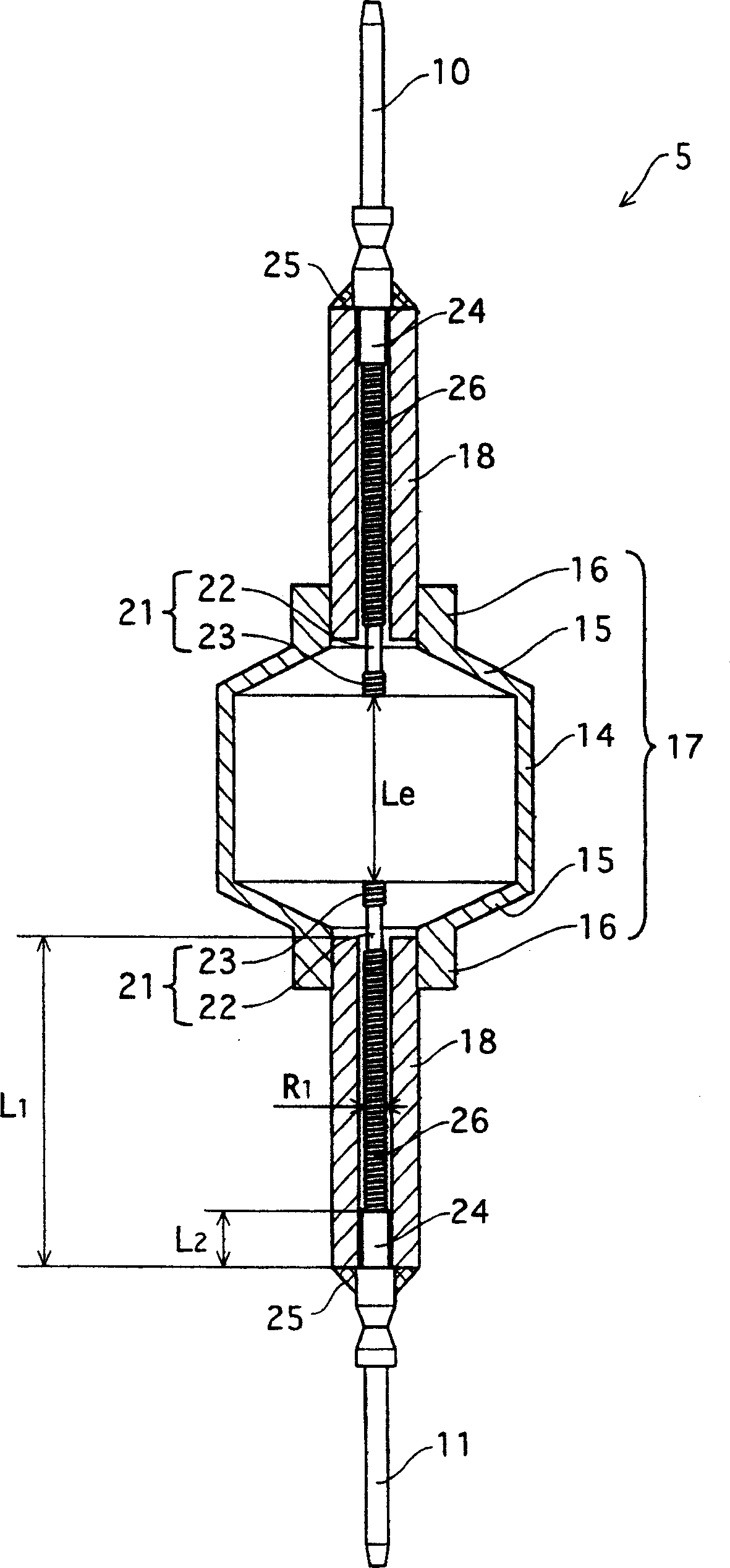
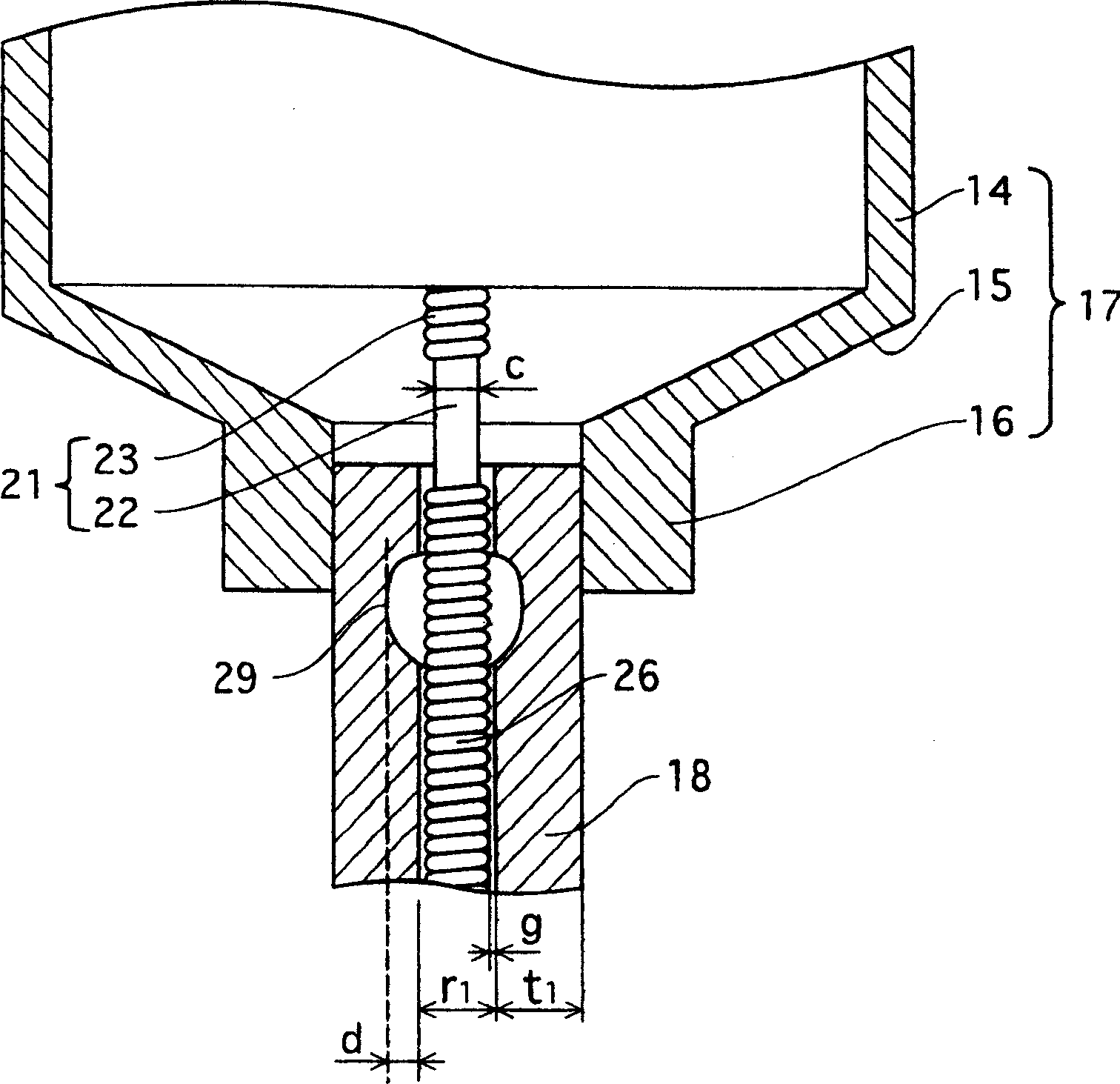
Metal halide lamp that has desired color characteristic and is prevented from non-lighting due to leakage of arc tube attributable to crack occurring at thin tube, and lighting apparatus adopting the metal halide lamp
Provided is a metal halide lamp capable of being dimmed, which is prevented from non-lighting due to leakage of an arc tube attributable to a crack occurring at thin tubes, as well as realizing a desired color characteristic. The metal halide lamp has an arc tube that includes an envelope made of translucent ceramic, a pair of electrodes, and metal halides, the metal halides including rare earth metal halide, sodium halide, and magnesium halide, the rare earth metal halide being at least one of dysprosium halide, thulium halide, holmium halide, cerium halide, and praseodymium halide, and the magnesium halide being at least one of magnesium iodide and magnesium bromide, where when a maximum lamp power P (W) is in a range of 70 W to 250 W, the following relations are satisfied: 0.0345A+0.0028B<0.0015P+0.0475; A≧0.021P+0.313; and B≧10.0, where A (mg) represents a total content of the metal halides, and B (mol %) represents a content ratio of the magnesium halide to the metal halides.
Owner:PANASONIC CORP
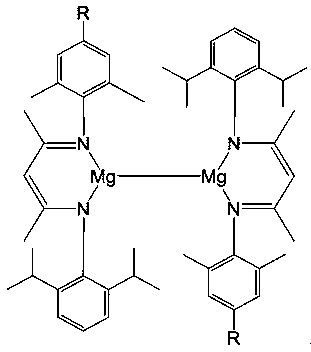
Asymmetric beta-diimine univalent magnesium complex as well as preparation method and application thereof in hydroboration of nitrile
InactiveCN107602594ASimple structureEasy to synthesizeOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsIodideAniline
The invention discloses an asymmetric beta-diimine univalent magnesium complex as well as a preparation method and an application thereof in a hydroboration reaction of nitrile. The preparation methodcomprises the steps as follows: firstly, acetylacetone and 2,6-diisopropyl aniline are condensed and then react with 2,4,6-trimethylaniline to produce an asymmetric beta-diimine ligand, then the asymmetric beta-diimine ligand reacts with the same amount of methylmagnesium iodide to produce magnesium iodide, finally, the magnesium iodide is reduced with excessive sodium, and the asymmetric beta-diimine univalent magnesium complex is obtained. The preparation method is simple, the synthesized asymmetric beta-diimine univalent magnesium complex has the high yield and has significant effects in the hydroboration reaction of nitrile, the reaction condition is mild, the reaction speed is high, the yield can reach 90% or above, and a catalyst has very high activity.
Owner:NANJING FORESTRY UNIV +1
Method for synthesizing 2-methylisoborneol
InactiveCN101531569AShort synthesis cycleLow costPreparation by hydrolysisAlkyl transferSimple Organic Compounds
The invention relates to a method for synthesizing a secondary metabolite of blue algae-2-methylisoborneol (the full name is 1,2,7,7-tetramethyl-outer-double ring-[2,2,1]-2-enanthol). The method comprises that d-camphor and organic compounds of magnesium (methyl magnesium iodide) are subjected to alkylation reaction to synthesize the 2-methylisoborneol, wherein the methyl magnesium iodide is prepared by the reaction of iodomethane and metal magnesium in absolute ethyl ether. The invention also relates to a method for removing impurities from the synthesized product and purifying and identifying the 2-methylisoborneol.
Owner:TONGJI UNIV
Asymmetric beta-diimine monovalent magnesium compound, preparation method and application thereof in aldehyde-ketone-boron hydrogenation reaction
ActiveCN107556196AHigh catalytic activitySimple structureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDiimineHydrogenation reaction
The invention discloses an unsymmetrical β-diimine monovalent magnesium compound, a preparation method thereof and an application in aldehyde and ketone hydroboration reactions. Its preparation method is as follows: first condense acetylacetone with different kinds of aromatic amines respectively to generate asymmetric β-diimine ligands, then react it with an equal amount of methylmagnesium iodide to generate magnesium iodide, and finally use excess The sodium reduction of unsymmetrical β-diimine monovalent magnesium compound. The preparation method of the invention is simple, and the synthesized asymmetric β-diimine monovalent magnesium compound has a remarkable effect in the hydroboration reaction of aldehydes and ketones, and the catalyst consumption is only 0.1%, the reaction speed is fast, the yield is very high, and it is highly in line with green Chemistry concept.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +1
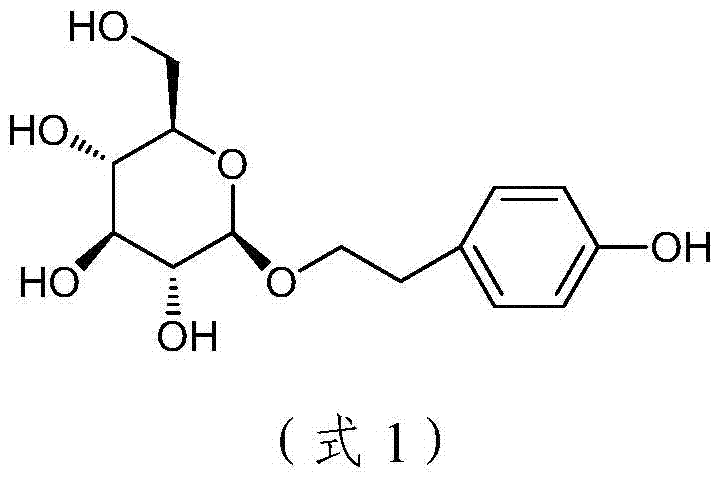
Method for catalytic synthesis of salidroside
ActiveCN104592321AHigh purification costHigh catalytic efficiencySugar derivativesSugar derivatives preparationSalidrosideOrganic base
The invention discloses a method for catalytic synthesis of salidroside. The method comprises the following steps: enabling total acetyl glucose to react with 4-benzyloxy-phenethyl alcohol under the action of a catalyst to obtain 2-(4-benzyloxyphenyl)ethyl-(2,3,4,6-O-tetra-acetyl)-beta-D-glucopyranoside, wherein the catalyst is tin chloride, zinc oxide, aluminum trichloride, boron fluoride or copper chloride, or a mixture of one of tin chloride, zinc oxide, aluminum trichloride, boron fluoride and copper chloride as well as magnesium fluoride, magnesium chloride, magnesium bromide or magnesium iodide; and removing an acetyl protective group from the product in the presence of an organic base to obtain 2-(4-benzyloxyphenyl)ethyl-beta-D-glucopyranoside, and introducing hydrogen into 2-(4-benzyloxyphenyl)ethyl-beta-D-glucopyranoside under the catalysis of palladium and carbon to perform reduction reaction so as to remove a benzyl protective group, thereby obtaining 2-(4-hydroxyphenyl)ethyl-beta-D-glucopyranoside. The method disclosed by the invention has the outstanding characteristics that the yield is high, the total acetyl glucose is subjected to three steps of reaction, the total yield can reach 63%, the process is simple, the cost is low, and the method is suitable for industrial mass production.
Owner:WUHAN SYNCHALLENGE UNIPHARM INC
Method for preparing alkaline type magnesium iodide crystal whiskers
InactiveCN107447256AMeet whisker requirementsPolycrystalline material growthFrom normal temperature solutionsMagnesium iodideWhiskers
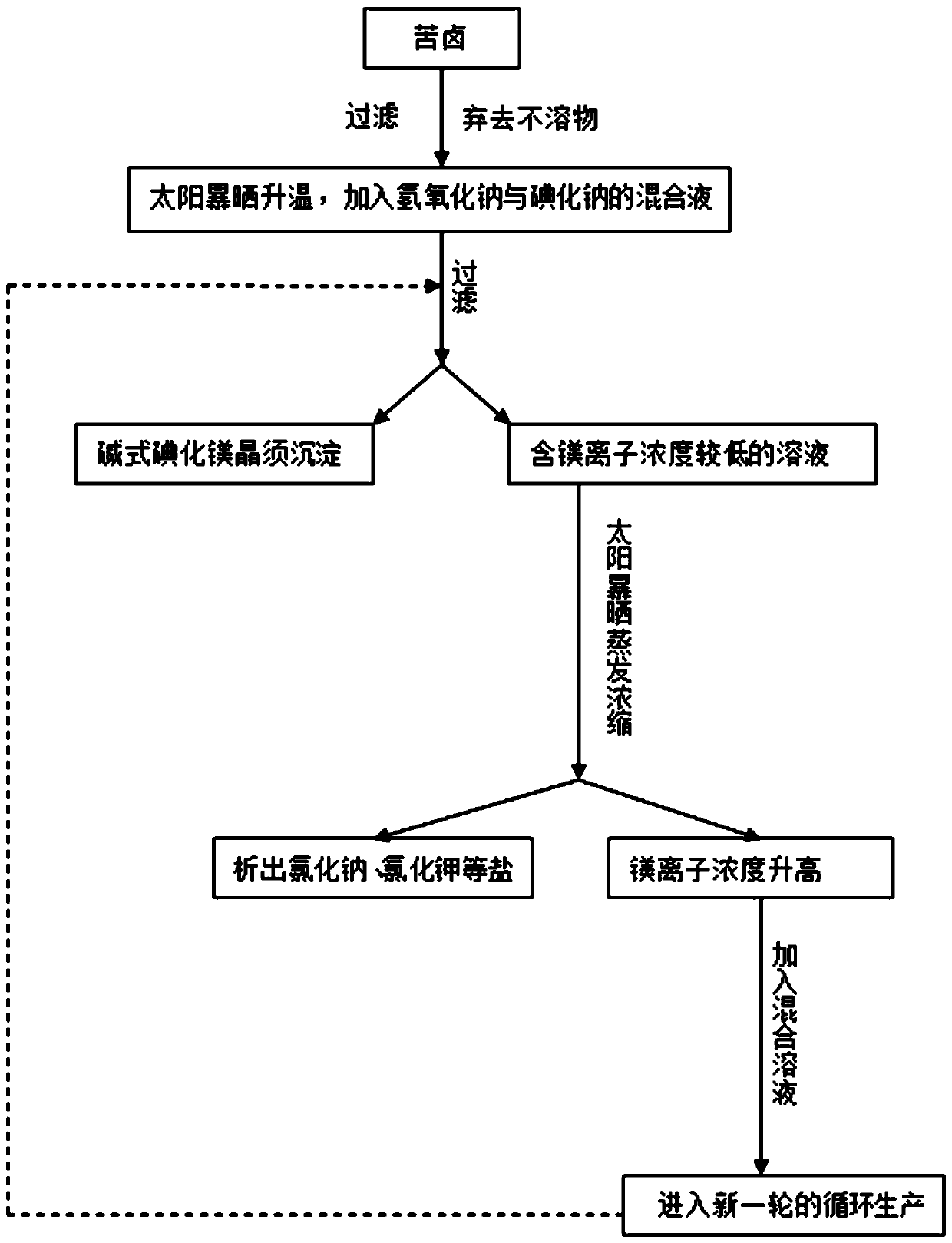
The invention discloses a method for preparing alkaline type magnesium iodide crystal whiskers. The method comprises the following steps: S1, taking bittern, filtering and discarding insoluble substances; S2, mixing a NaI solution with concentration of 3 mol / L with a bittern solution treated in step S1 in a volume ratio of 3 to 1 to obtain a mixed solution 1; S3, heating the mixed solution 1 to 30 DEG C, then dropwise adding a NaOH solution with concentration of 0.8 mol / L into the mixed solution 1 under a stirring state to obtain a mixed solution 2, wherein a volume ratio of the mixed solution 1 to the NaOH solution with concentration of 0.8 mol / L is (2.5-3.5) to 1; and S4, continuously ageing the mixed solution 2 for 55 hours at a temperature of 35 DEG C, filtering and collecting precipitates, and drying the precipitates to obtain the alkaline type magnesium iodide crystal whiskers.
Owner:LINGNAN NORMAL UNIV
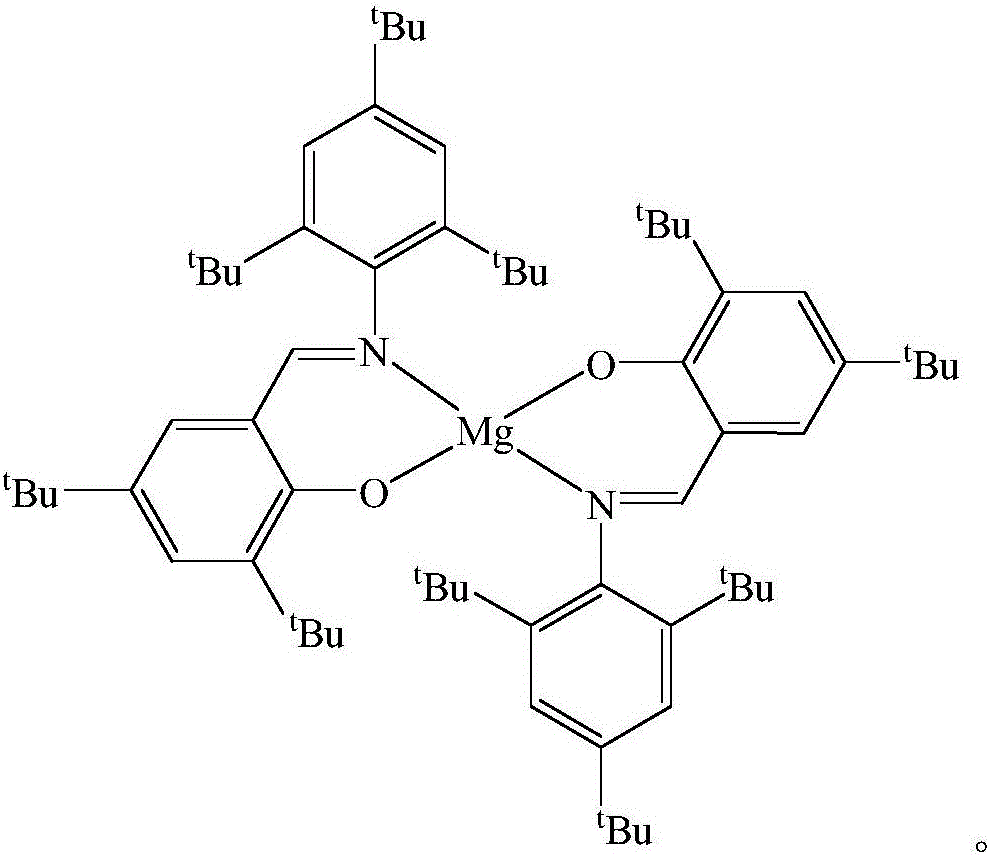
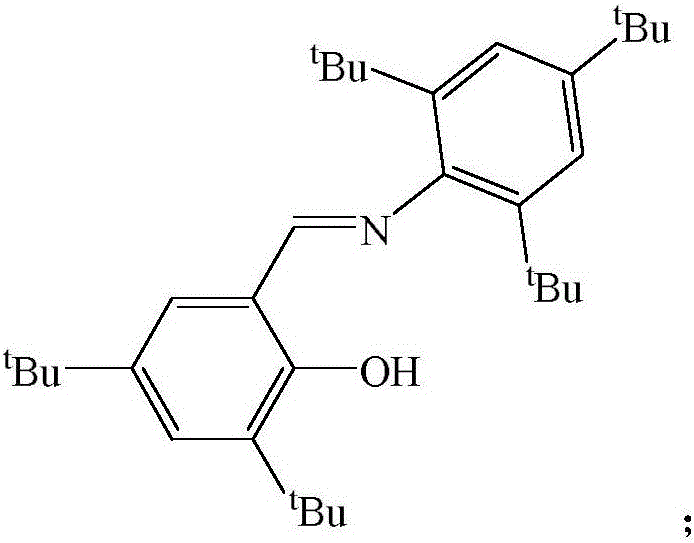
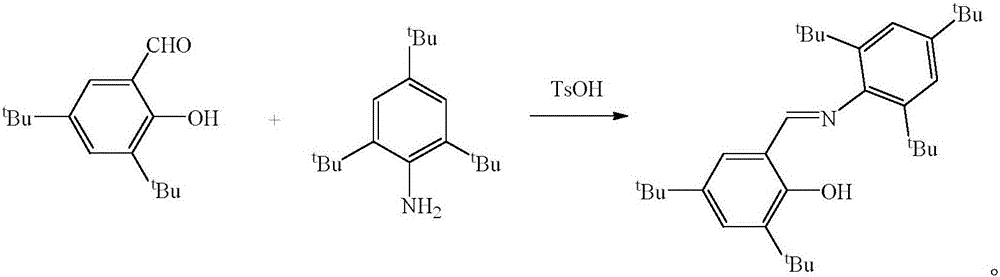
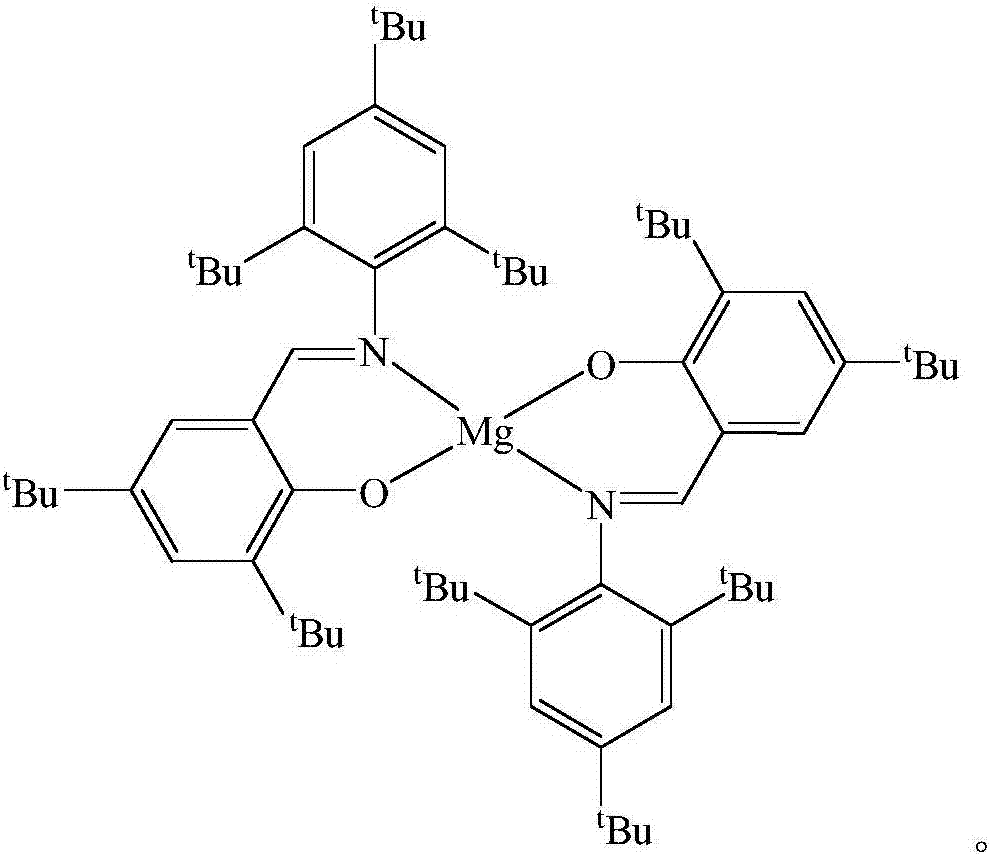
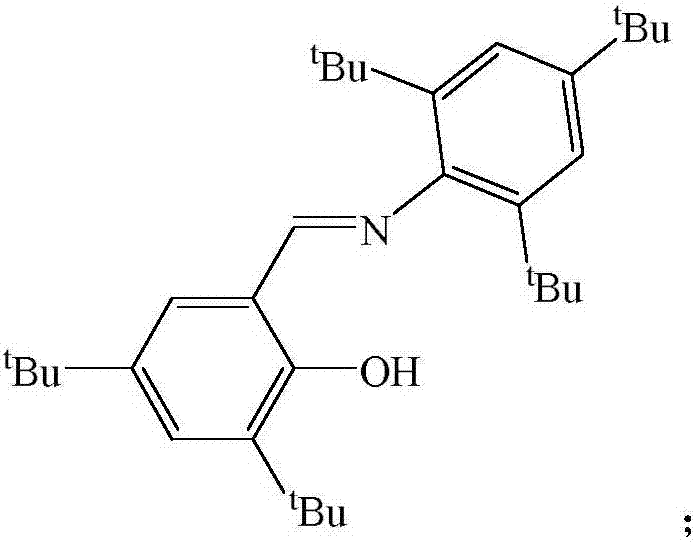
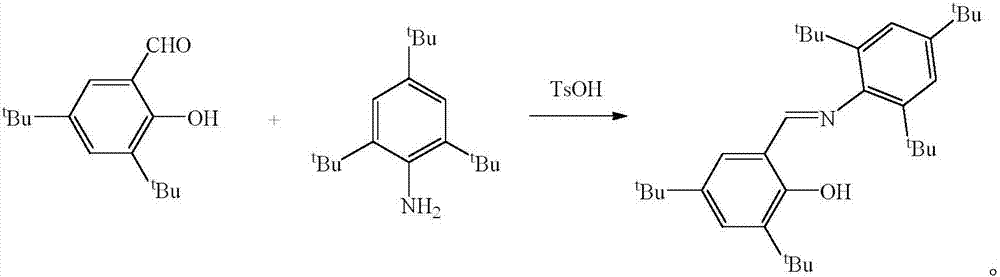
Schiff base magnesium organometallic compound, and preparation method and application thereof
InactiveCN106242996AEasy to purifyLow toxicitySilicon organic compoundsOxygen-containing compound preparationIodideMagnesium iodide
The invention discloses a Schiff base magnesium organometallic compound, and a preparation method and an application thereof. The preparation method comprises the following steps: 1, heating and refluxing 2,4,6-tri-tert-butylaniline, 3,5-di-tert-butylsalicylaldehyde and p-toluenesulfonic acid in ethanol until reaction is completed to precipitate a large amount of pale yellow crystals which are 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base; 2, respectively reacting the 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base and 2-(2,6-di(diphenylmethyl)-4-isopropyl)-3,5-di-tert-butyl Schiff base with methylmagnesium iodide under waterless anoxic conditions to obtain a large amount of colorless crystals which are 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base magnesium metal compound and 2-(2,6-di(diphenylmethyl)-4-isopropyl)-3,5-di-tert-butyl Schiff base magnesium iodide respectively; and 3, reacting the 2-(2,6-di(diphenylmethyl)-4-isopropyl)-3,5-di-tert-butyl Schiff base magnesium iodide with metallic sodium to obtain pale yellow crystals which are 2-(2,6-di(diphenylmethyl)-4-isopropyl)-3,5-di-tert-butyl sodium magnesium bi-metal compound.
Owner:南京芬纽克新材料有限公司
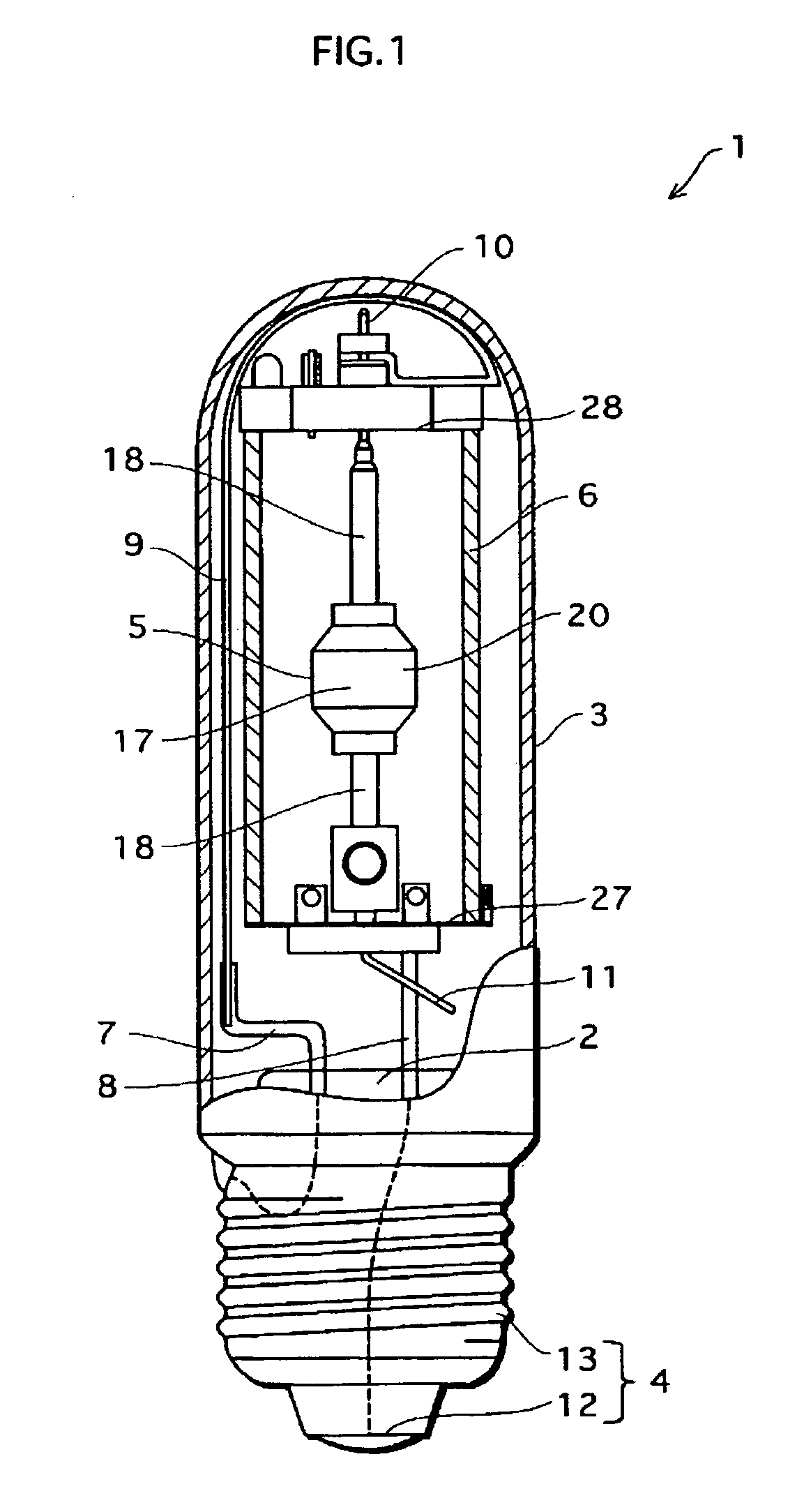
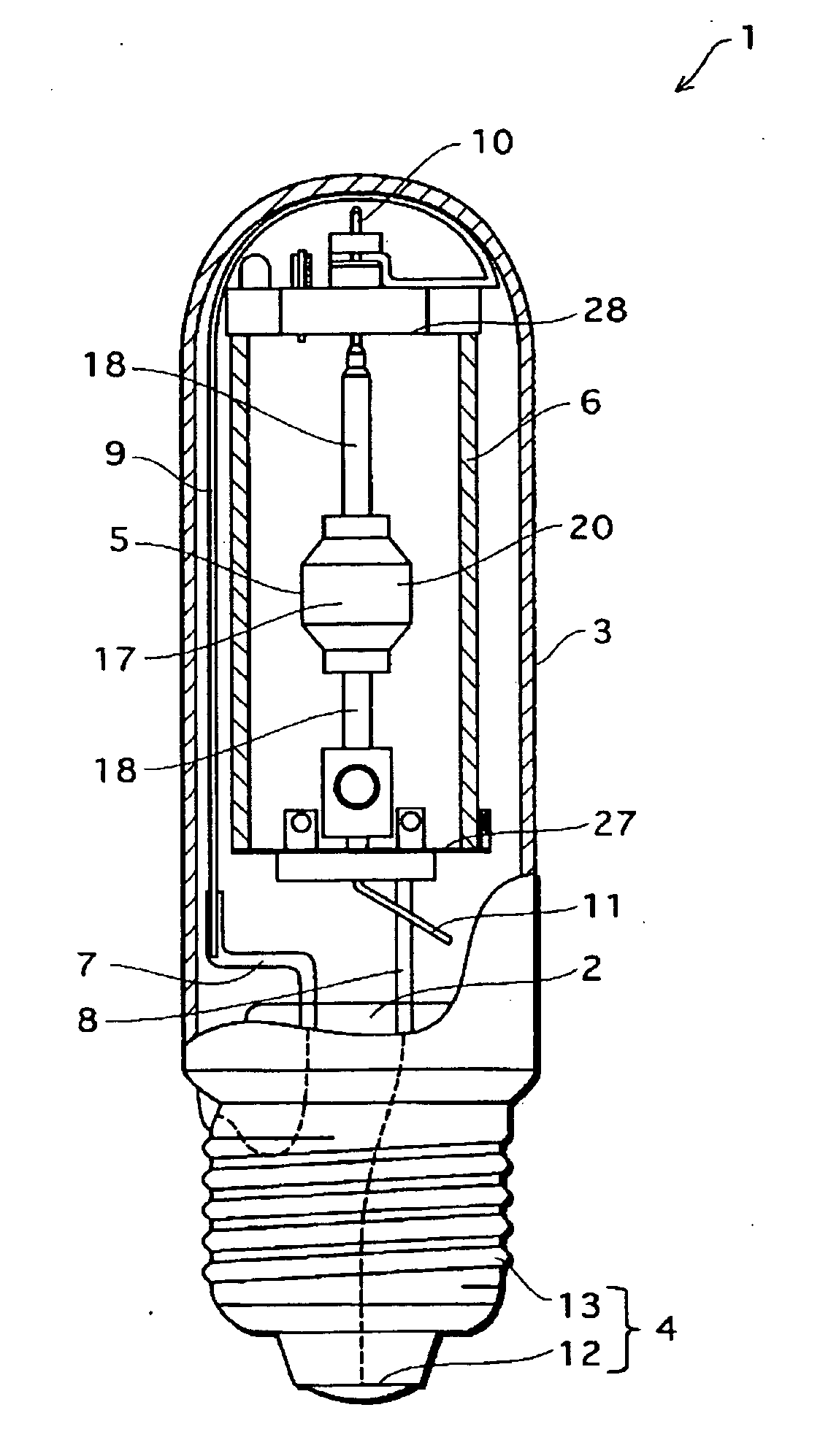
Metal halide lamp and lighting device using same
InactiveCN1801453APrevent non-luminous phenomenonAchieve color renderingPoint-like light sourceHigh-pressure discharge lampsEffect lightCerium
Provided is a metal halide lamp capable of being dimmed, which is prevented from non-lighting due to leakage of an arc tube attributable to a crack occurring at thin tubes, as well as realizing a desired color characteristic. The metal halide lamp has an arc tube that includes an envelope made of translucent ceramic, a pair of electrodes, and metal halides, the metal halides including rare earth metal halide, sodium halide, and magnesium halide, the rare earth metal halide being at least one of dysprosium halide, thulium halide, holmium halide, cerium halide, and praseodymium halide, and the magnesium halide being at least one of magnesium iodide and magnesium bromide, where when a maximum lamp power P (W) is in a range of 70 W to 250 W, the following relations are satisfied: <?in-line-formulae description="In-line Formulae" end="lead"?>0.0345A+0.0028B<0.0015P+0.0475;<?in-line-formulae description="In-line Formulae" end="tail"?> <?in-line-formulae description="In-line Formulae" end="lead"?>A>=0.021P+0.313; and<?in-line-formulae description="In-line Formulae" end="tail"?> <?in-line-formulae description="In-line Formulae" end="lead"?>B>=10.0,<?in-line-formulae description="In-line Formulae" end="tail"?> where A (mg) represents a total content of the metal halides, and B (mol %) represents a content ratio of the magnesium halide to the metal halides.
Owner:PANASONIC CORP
Fluorine-containing organosilicone monomer and preparation method thereof
ActiveCN104829641AHigh fluorine contentNot cumulatively toxicGroup 4/14 element organic compoundsCarbon chainMonomer
The invention discloses a fluorine-containing organosilicone monomer and a preparation method thereof. The method is characterized in that the method comprises the steps that 3-tridecafluorohexyl propylene and tridecafluoro-1-iodohexane are taken as starting materials, and undergo a single electron transfer addition reaction to synthesize 1,3-bi-(tridecafluorohexyl)-2-iodopropane; 1,3-bi-(tridecafluorohexyl)-2-iodopropane reacts with magnesium metal to prepare 1,3-bi-(tridecafluorohexyl)-2-propyl magnesium iodide which further reacts with silicon tetrachloride to form fluorine-containing alkyl-substituted chlorosilane; and a bi-[1,3-bi-(tridecafluorohexyl) isopropyl] dichloro-silicohydride product is obtained by recification. In a molecular structure of the product, perfluoroalkyl is short-chain perfluoroalkyl; the length of a carbon chain is equal to 6; the product is not degraded difficultly, has no cumulative toxicity, and is an environment-friendly monomer for synthesizing fluorine-containing polysiloxane. The preparation and reaction conditions of the fluorine-containing organosilicone monomer are mild; a technique is simple and convenient; the raw materials are easy to obtain; and industrialized production, and popularization and application are facilitated.
Owner:JIANGSU HUAJIA SILK
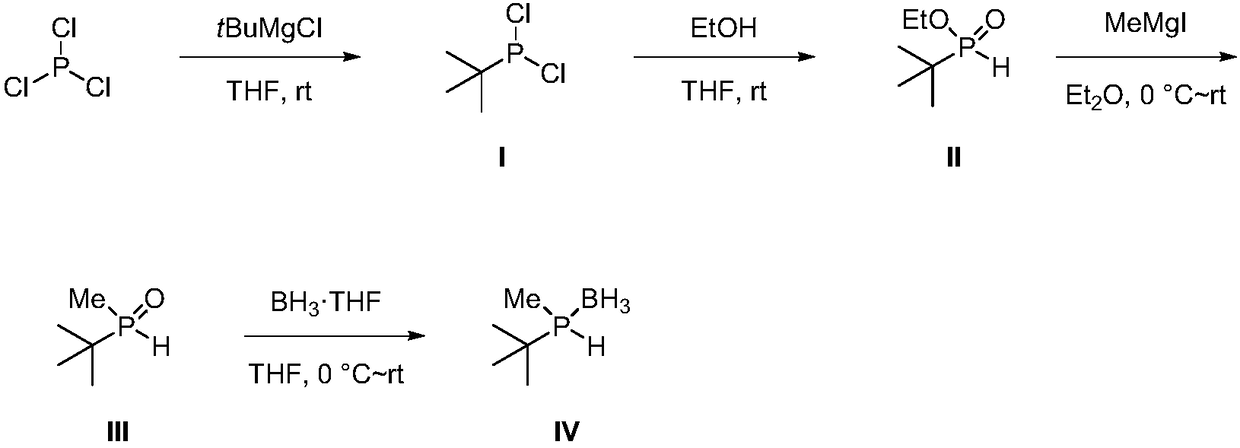
Phosphorus chiral important intermediate preparation method
ActiveCN108409784AShort process routeSimple and fast operationGroup 5/15 element organic compoundsGrignard reagentTert-Butyl chloride
The invention discloses a phosphorus chiral important intermediate preparation method, which comprises: carrying out a substitution reaction on phosphorus trichloride and tert-butyl magnesium chlorideto obtain tert-butyl phosphine dichloride I; carrying out an esterification reaction on the tert-butyl phosphine dichloride I and ethanol to obtain tert-butyl ethyl phosphite II; carrying out a methylation reaction on the tert-butyl ethyl phosphite II under the action of a methyl Grignard reagent methyl magnesium iodide to generate methyl tert-butyl phosphine oxide III; and obtaining a methyl tert-butyl phosphine hydrogen compound VI protected with borane through a borane reduction one-pot method. According to the present invention, the method has advantages of low-cost and easily-available raw material, short route, simple operation and good atomic economy, and provides the simple and easy-performing route for the preparation of the borane methyl tert-butyl phosphine hydrogen.
Owner:SHANGHAI JIAO TONG UNIV
A kind of Schiff base magnesium organic metal compound and its preparation method and application
InactiveCN106242996BEasy to purifyLow toxicitySilicon organic compoundsOxygen-containing compound preparationIodideMagnesium iodide
The invention discloses a Schiff base magnesium organometallic compound, and a preparation method and an application thereof. The preparation method comprises the following steps: 1, heating and refluxing 2,4,6-tri-tert-butylaniline, 3,5-di-tert-butylsalicylaldehyde and p-toluenesulfonic acid in ethanol until reaction is completed to precipitate a large amount of pale yellow crystals which are 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base; 2, respectively reacting the 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base and 2-(2,6-di(diphenylmethyl)-4-isopropyl)-3,5-di-tert-butyl Schiff base with methylmagnesium iodide under waterless anoxic conditions to obtain a large amount of colorless crystals which are 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base magnesium metal compound and 2-(2,6-di(diphenylmethyl)-4-isopropyl)-3,5-di-tert-butyl Schiff base magnesium iodide respectively; and 3, reacting the 2-(2,6-di(diphenylmethyl)-4-isopropyl)-3,5-di-tert-butyl Schiff base magnesium iodide with metallic sodium to obtain pale yellow crystals which are 2-(2,6-di(diphenylmethyl)-4-isopropyl)-3,5-di-tert-butyl sodium magnesium bi-metal compound.
Owner:南京芬纽克新材料有限公司
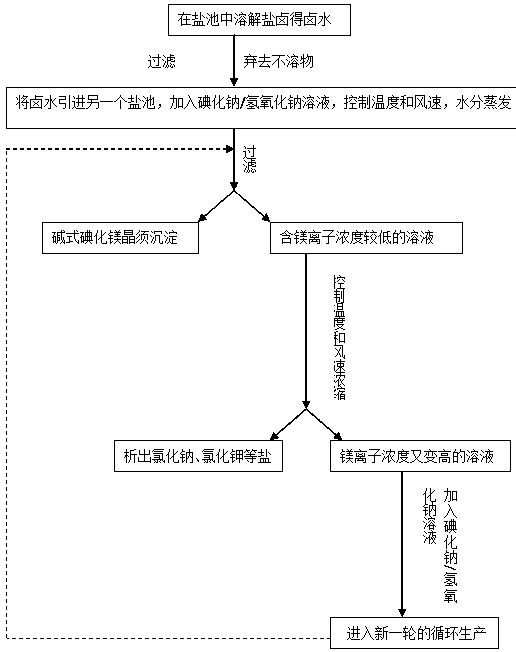
Method for producing alkali magnesium iodide crystal whisker from bittern of dried lake
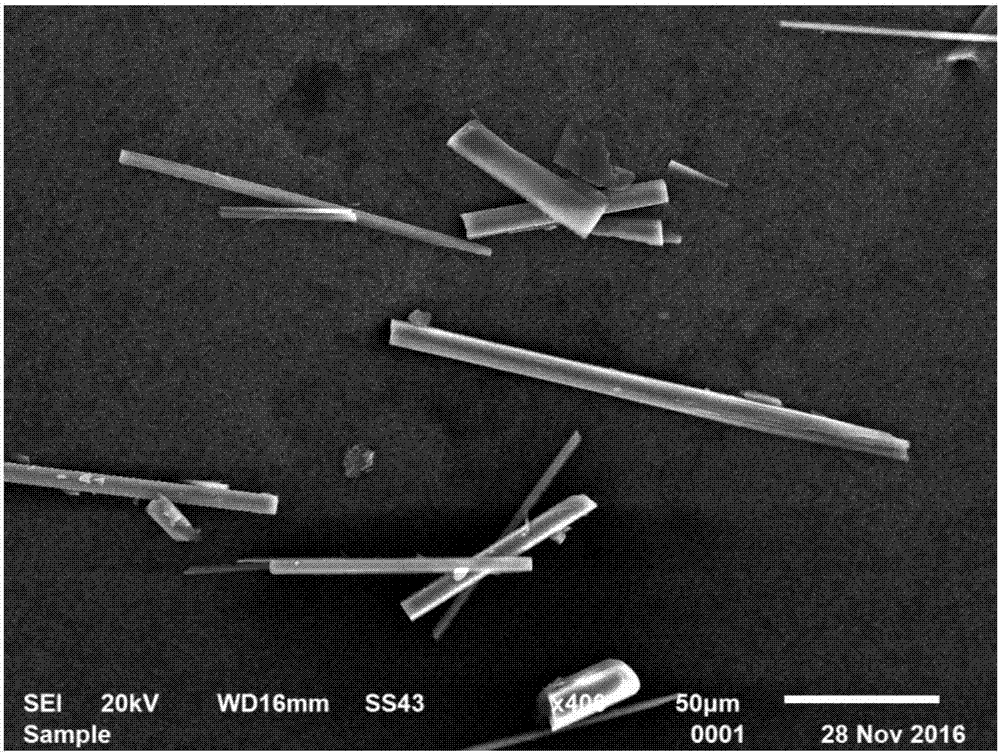
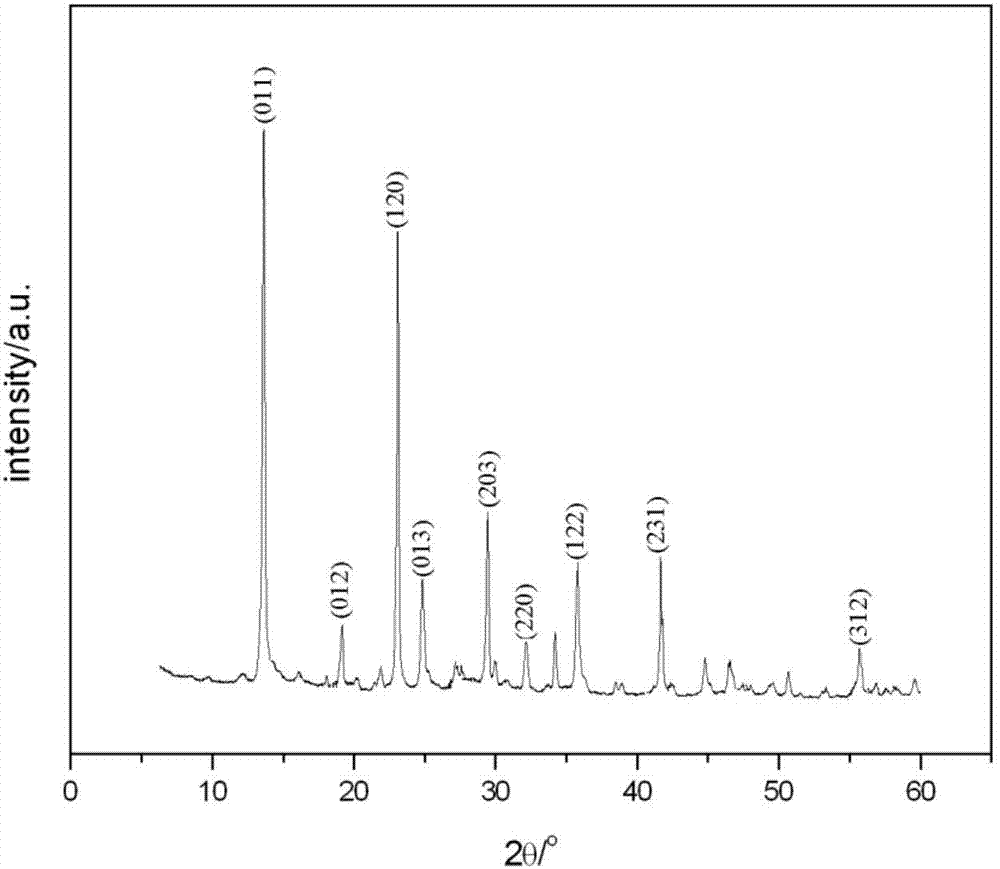
InactiveCN107935001ASmall changes in local concentrationGood dispersionMagnesium halidesMagnesium iodideMagnesium ion
The invention relates to a method for producing alkali magnesium iodide crystal whiskers from bittern of a dried lake. The method comprises the following steps: S1, dissolving bittern into water so asto obtain brine, wherein the concentration of magnesium ions in the brine is 2.3-2.9mol / L; S2, mixing the brine with an iodate / strong alkali solution, wherein the concentration of iodate in the iodate / strong alkali solution is 2.3-2.9mol / L, the concentration of the strong alkali is 0.18-0.22mol / L, and the volume ratio of the brine to the iodate / strong alkali solution is (0.98-1.02):1; S3, performing moisture evaporation on the solution obtained in the step S2 at minus 1 DEG C to 51 DEG C at an air speed of 4-5m / s for 67-77 hours, filtering so as to obtain precipitate, namely the alkali magnesium iodide crystal whiskers, and recycling obtained filtrate for next circulation treatment. The draw ratio of the alkali magnesium iodide crystal whiskers prepared by using the method provided by theinvention is (20-30):1, and the alkali magnesium iodide crystal whiskers are good in dispersability and high in quality.
Owner:LINGNAN NORMAL UNIV
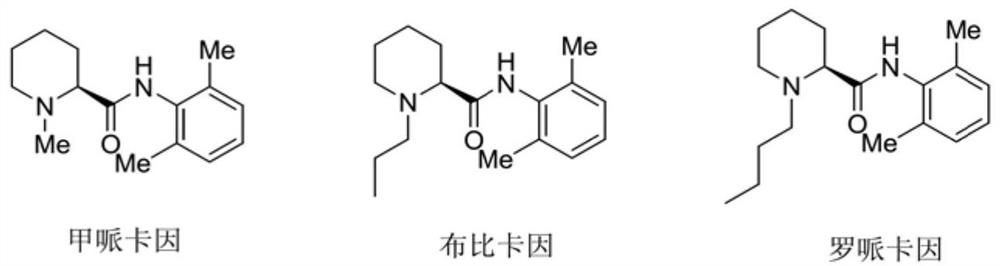
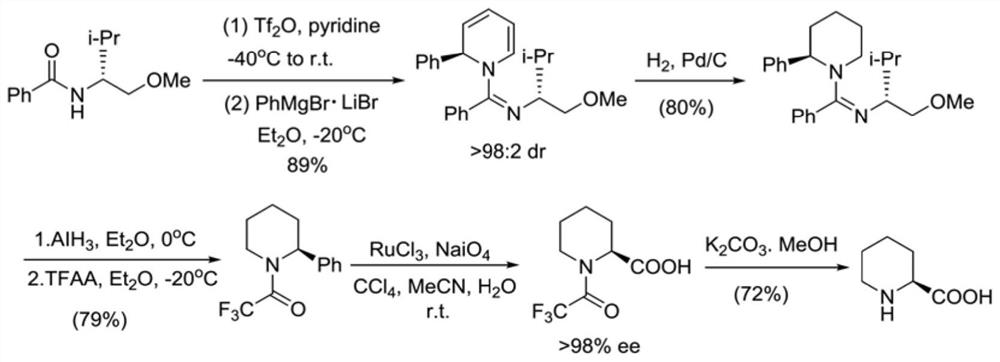
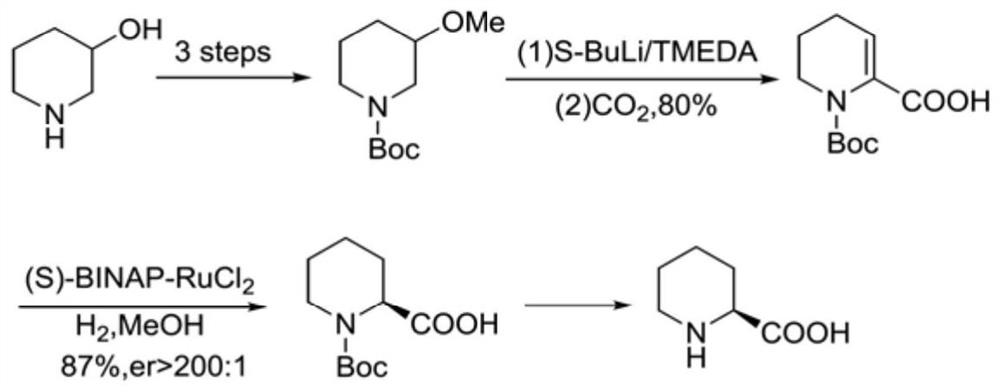
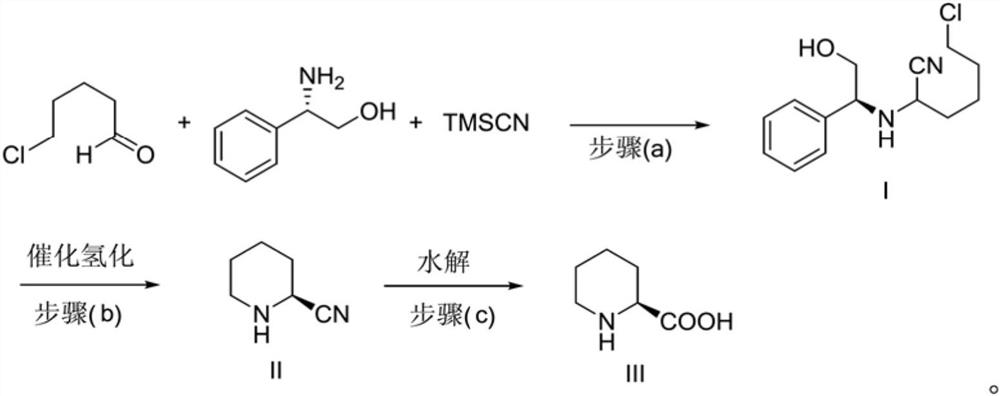
A kind of preparation method of caine drug intermediate (s)-2-piperidinecarboxylic acid
The invention discloses a preparation method of a caine drug intermediate (S)-2-piperidinecarboxylic acid, which specifically comprises the following steps: a) preparing 5-chlorovaleraldehyde, L-phenylene glycol and trimethylsilyl cyanide Carry out one-pot reaction under the effect of catalyst A to obtain the compound shown in formula (I); Described catalyst A is magnesium diiodide, magnesium dibromide, magnesium perchlorate; (b) formula (I) The compound catalytic hydrogenation reaction shown obtains (S)-2-cyanopiperidine shown in formula (II); (c) the compound hydrolysis shown in formula (II) obtains (S)-2 shown in formula (III) ‑piperidinecarboxylic acid. The preparation method utilizes cheap and easy-to-obtain organic raw materials, and has the advantages of simple operation, mild reaction conditions, good stereoselectivity, high yield and the like.
Owner:ZHEJIANG UNIV OF TECH
A kind of unsymmetrical β-diimine monovalent magnesium complex and its preparation method and application in nitrile hydroboration
InactiveCN107602594BSimple structureEasy to synthesizeOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsIodideAniline
The invention discloses an asymmetric beta-diimine univalent magnesium complex as well as a preparation method and an application thereof in a hydroboration reaction of nitrile. The preparation methodcomprises the steps as follows: firstly, acetylacetone and 2,6-diisopropyl aniline are condensed and then react with 2,4,6-trimethylaniline to produce an asymmetric beta-diimine ligand, then the asymmetric beta-diimine ligand reacts with the same amount of methylmagnesium iodide to produce magnesium iodide, finally, the magnesium iodide is reduced with excessive sodium, and the asymmetric beta-diimine univalent magnesium complex is obtained. The preparation method is simple, the synthesized asymmetric beta-diimine univalent magnesium complex has the high yield and has significant effects in the hydroboration reaction of nitrile, the reaction condition is mild, the reaction speed is high, the yield can reach 90% or above, and a catalyst has very high activity.
Owner:NANJING FORESTRY UNIV +1
A kind of passive magnesium chemical phase analysis method
InactiveCN104237227BEfficient separationFast wayMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationEthylene diamineAlcohol
The invention discloses a chemical phase analysis method for passivated magnesium. The chemical phase analysis method comprises the following steps: utilizing the characteristic that the magnesium metal in a specimen can take a reaction with iodine to generate magnesium iodide to be dissolved in methyl alcohol, so as to effectively separate out the magnesium metal from the specimen, enabling the magnesium oxide in the precipitate to take a reaction with nitric acid, putting the reactant in a solution, respectively carrying out EDTA (Ethylene Diamine Tetraacetic Acid) developing titration on the solution dissolved with the magnesium metal or magnesium oxide, so as to obtain the content of the magnesium metal and the magnesium oxide. The chemical phase analysis method disclosed by the invention is quick, accurate, efficient, and simple in operation, and has better practicability.
Owner:武汉钢铁有限公司
A kind of unsymmetrical β-diimine monovalent magnesium compound and its preparation method and application in hydroboration reaction of aldehydes and ketones
ActiveCN107556196BHigh catalytic activitySimple structureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystIodide
The invention discloses an asymmetric beta-diimine monovalent magnesium compound, a preparation method and an application thereof in aldehyde-ketone-boron hydrogenation reaction. The preparation method comprises the following steps: firstly condensing acetylacetone respectively with different types of aromatic amine to generate asymmetric beta-diimine ligand, then reacting the asymmetric beta-diimine ligand with equivalent magnesium methyl iodide to generate iodide of magnesium, and finally using excessive sodium for reduction to obtain the asymmetric beta-diimine monovalent magnesium compound. The preparation method disclosed by the invention is simple, the synthesized asymmetric beta-diimine monovalent magnesium compound has an obvious effect in aldehyde-ketone-boron hydrogenation reaction, the use amount of a catalyst is only 0.1%, the reaction speed is high, the yield is very high, and the idea of green chemistry is highly conformed.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +1
Recycling phase change material
InactiveCN107177347APhase change energy storage effect is goodGood effectHeat-exchange elementsSodium acetatePotassium fluoride
The invention discloses a phase change energy storage material capable of recycling, which comprises calcium dichloride, potassium fluoride, manganese nitrate, magnesium iodide, sodium sulfate, sodium acetate, lithium chlorate, sodium carbonate, Water, the proportion of each component of the recyclable phase change energy storage material is: in parts by weight, 5-10 parts of calcium dichloride, 8-12 parts of potassium fluoride, 13-17 parts of manganese nitrate, Magnesium iodide 11-15 parts, sodium sulfate 7-12 parts, sodium acetate 3-7 parts, lithium chlorate 10-15 parts, sodium carbonate 4-8 parts, water 30-35 parts. Through the above method, the present invention can achieve a very good phase change energy storage effect through the compound use of several inorganic substances, the material can be recycled, and it still has a very good effect after multiple cycles, saving the cost of use. Energy is saved.
Owner:PIONEER ENERGY JIANGSU
Method for preparing basic magnesium iodine crystal whiskers from bittern by using solar energy
InactiveCN107687020AIncrease productionImprove qualityPolycrystalline material growthFrom normal temperature solutionsPollutantSunlight
The invention discloses a method for preparing basic magnesium iodine crystal whiskers from bittern by using solar energy. The method comprises the following steps of S1, filtering insoluble matters in the bittern; S2, leading the filtered bittern into a salt field or a transparent container, and exposing under sunlight; S3, adding a NaI (sodium iodine) and NaOH (sodium hydroxide) mixed solution into the exposed bittern, continuing to expose for 2 to 4 days under the sunlight, and filtering the bittern, so as to obtain the basic magnesium iodine crystal whiskers. The method has the advantagesthat the basic magnesium iodine crystal whiskers are prepared from the bittern by using the solar energy and the self-cleanliness of the crystal whiskers, the operation is simple, and the complicatedequipment is not needed; the energy consumption is low, the pollutant and public hazard are avoided, and the salt lake resource is utilized in clean and environment-friendly ways; the output of the crystal whiskers is high, and the quality is high; the method is suitable for preparing the magnesium salt crystal whiskers in a large scale way in the actual production, and the application prospect islarger.
Owner:LINGNAN NORMAL UNIV
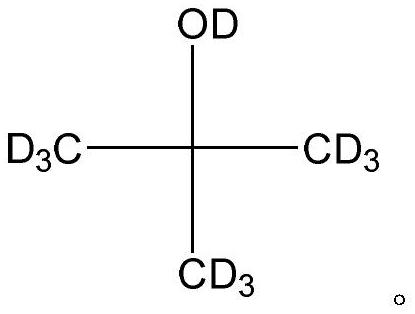
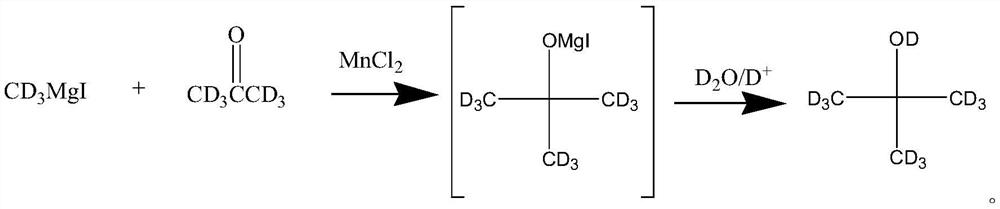
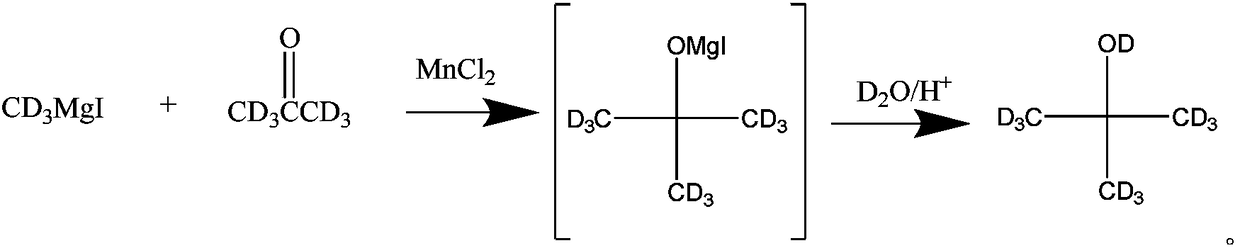
A kind of preparation method of all deuterated tert-butanol
ActiveCN108164393BSimple production processIncrease abundanceOrganic chemistry methodsMagnesium organic compoundsGrignard reactionHydrolysis
The invention discloses a total-deuteration tert butyl alcohol preparation method which comprises the following steps: firstly, making deuterium methyl magnesium iodide and deuteration acetone performGrignard reaction in anhydrous tetrahydrofuran under the existence of anhydrous manganese chloride; then hydrolyzing a reaction product under the acid condition to generate total-deuteration tert butyl alcohol, wherein the acid condition is formed by a heavy water solution which deuteration acid is added in. The total-deuteration tert butyl alcohol preparation method disclosed by the invention has the advantages of moderate reaction condition, high reaction yield, ability in obtaining abundant total-deuteration tert butyl alcohol and simpleness in technological operation.
Owner:上海枢炬化工有限公司
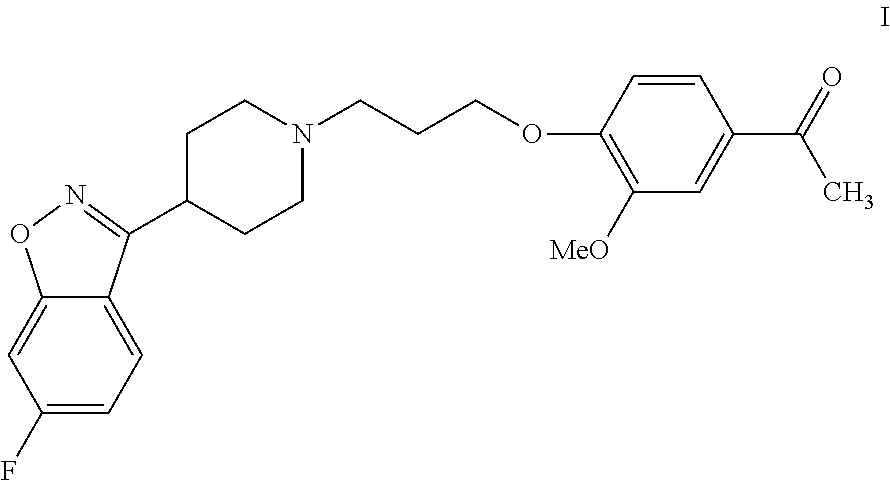
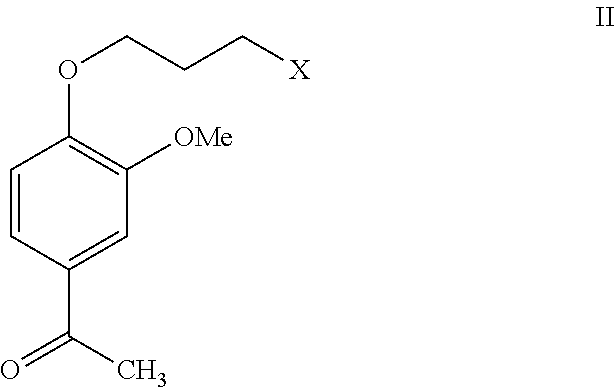
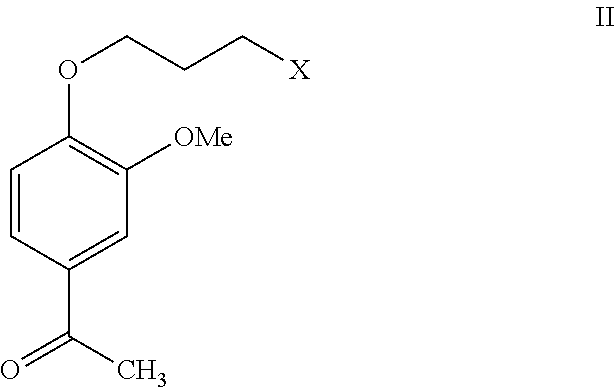
Process for the preparation of iloperidone using a novel intermediate
InactiveUS20120220776A1Speed up the processOrganic compound preparationCarbonyl compound preparation by oxidationEtherMagnesium iodide
The present invention provides a novel process for the preparation of iloperidone using a novel intermediate. Thus, for example, 4-(3-chloropropoxy)-3-methoxybenzaldehyde is reacted with methyl magnesium iodide in ether and the reaction mass is heated for 6 hours at reflux temperature, the resulting mass is cooled to ambient temperature and then poured into a mixture of ice, water and dilute hydrochloric acid to produce 1-[4-(3-chloropropoxy)-3-methoxyphenyl]ethanol, which is then subsequently converted to iloperidone.
Owner:SYMED LABS
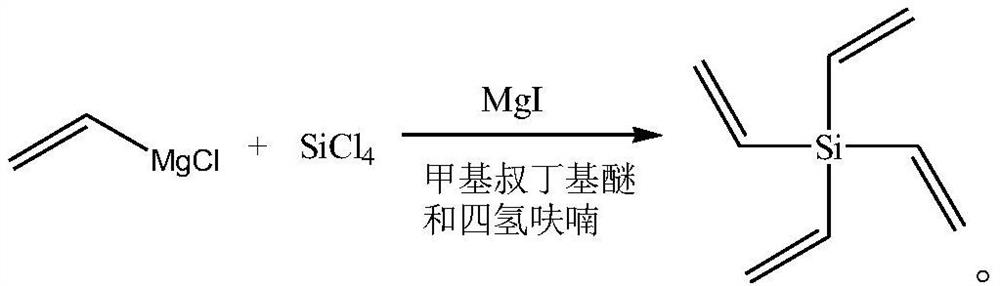
Synthesis method of tetraethylene silane
PendingCN114478611APromote Grignard reactionThorough responseSilicon organic compoundsElectrolytic agentPtru catalyst
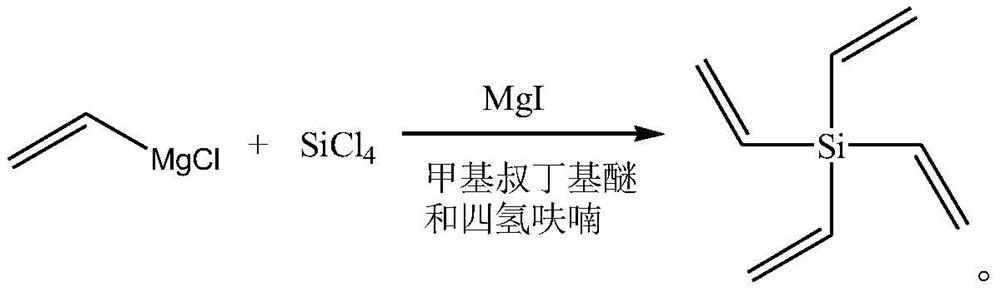
The invention discloses a synthesis method of tetraethylene silane, and relates to the technical field of battery electrolyte additives, and the synthesis method comprises the following steps: under the protection of an inert gas, dissolving tetrachlorosilane in methyl tert-butyl ether, adding a catalyst magnesium iodide, slowly dropwise adding a vinyl magnesium chloride solution into the obtained system, carrying out a Grignard reaction, and after the reaction is completed, carrying out a reaction to obtain the tetraethylene silane. And adding water for quenching, performing phase splitting, and performing vacuum concentration on the obtained organic phase to obtain the tetraethylene silane. The synthesis method provided by the invention is simple to operate, few in reaction steps and easy to purify, the product yield and the product purity in the production process are ensured to be maximized by controlling reaction conditions in each stage, and the product yield is further improved by reasonably controlling the dosage of reaction raw materials and the reaction process.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD +1
Chemical phase analysis method for passivated magnesium
InactiveCN104237227AEfficient separationFast wayMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationEthylene diamineAlcohol
The invention discloses a chemical phase analysis method for passivated magnesium. The chemical phase analysis method comprises the following steps: utilizing the characteristic that the magnesium metal in a specimen can take a reaction with iodine to generate magnesium iodide to be dissolved in methyl alcohol, so as to effectively separate out the magnesium metal from the specimen, enabling the magnesium oxide in the precipitate to take a reaction with nitric acid, putting the reactant in a solution, respectively carrying out EDTA (Ethylene Diamine Tetraacetic Acid) developing titration on the solution dissolved with the magnesium metal or magnesium oxide, so as to obtain the content of the magnesium metal and the magnesium oxide. The chemical phase analysis method disclosed by the invention is quick, accurate, efficient, and simple in operation, and has better practicability.
Owner:武汉钢铁有限公司
Catalytic synthesis method of salidroside
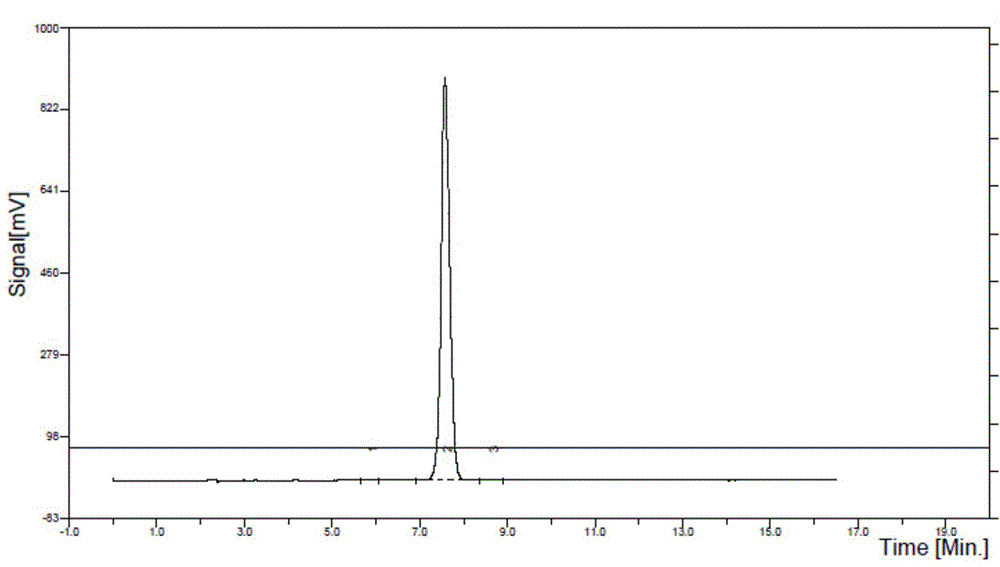
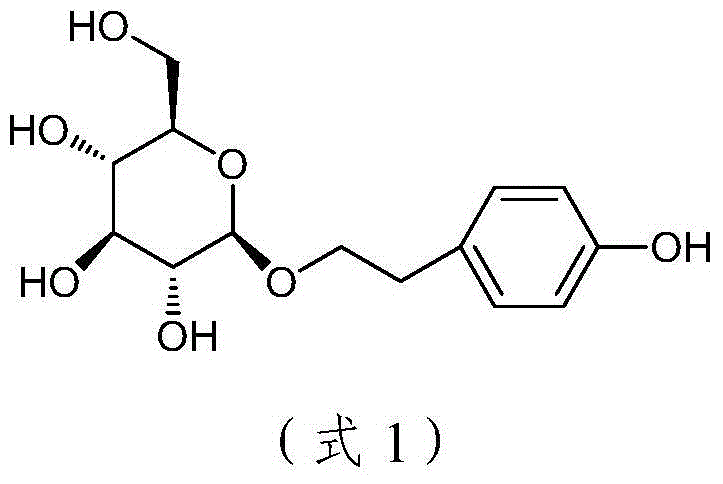
ActiveCN104592321BHigh purification costHigh catalytic efficiencySugar derivativesSugar derivatives preparationSalidrosidePhenethyl alcohol
Disclosed is a method for catalytic synthesis of salidroside. The method comprises: enabling total acetyl glucose to react with 4-benzyloxy-phenethyl alcohol under the action of a catalyst to obtain 2-(4-benzyloxyphenyl)ethyl-(2,3,4,6-O-tetra-acetyl)-β-D-glucopyranoside, the catalyst being tin chloride, zinc oxide, aluminum trichloride, boron fluoride or copper chloride, or a mixture formed by mixing one of tin chloride, zinc oxide, aluminum trichloride, boron fluoride and copper chloride with magnesium fluoride, magnesium chloride, magnesium bromide or magnesium iodide; and removing an acetyl protective group from the product in the presence of an organic base to obtain 2-(4-benzyloxyphenyl)ethyl-β-D-glucopyranoside, and introducing hydrogen into 2-(4-benzyloxyphenyl)ethyl-β-D-glucopyranoside under the catalysis of palladium and carbon to perform a reduction reaction so as to remove a benzyl protective group, thereby obtaining 2-(4-hydroxyphenyl)ethyl-β-D-glucopyranoside. The present invention has the outstanding characteristics that the yield is high, the total acetyl glucose is subjected to three steps of reactions, the total yield can reach 63%, the process is simple, the cost is low, and the present invention is suitable for industrial mass production.
Owner:WUHAN SYNCHALLENGE UNIPHARM INC
Preparation method of caine drug intermediate (S)-2-piperidinecarboxylic acid
The invention discloses a preparation method of a caine drug intermediate (S)-2-piperidinecarboxylic acid. The preparation method specifically comprises the following steps of a) carrying out a one-pot reaction on 5-chlorovaleraldehyde, L-phenylglycinol and trimethylsilyl cyanide under the action of a catalyst A to obtain a compound as shown in a formula (I), wherein the catalyst A is magnesium diiodide, magnesium dibromide and magnesium perchlorate, (b) carrying out catalytic hydrogenation reaction on the compound as shown in the formula (I) to obtain (S)-2-cyano piperidine as shown in a formula (II), and (c) hydrolyzing the compound as shown in the formula (II) to obtain (S)-2-piperidinecarboxylic acid as shown in a formula (III). The preparation method utilizes cheap and easily available organic raw materials, and has the advantages of simple operation, mild reaction conditions, good stereoselectivity, high yield and the like.
Owner:ZHEJIANG UNIV OF TECH
Total-deuteration tert butyl alcohol preparation method
ActiveCN108164393ASimple production processIncrease abundanceOrganic chemistry methodsMagnesium organic compoundsChlorideMagnesium iodide
The invention discloses a total-deuteration tert butyl alcohol preparation method which comprises the following steps: firstly, making deuterium methyl magnesium iodide and deuteration acetone performGrignard reaction in anhydrous tetrahydrofuran under the existence of anhydrous manganese chloride; then hydrolyzing a reaction product under the acid condition to generate total-deuteration tert butyl alcohol, wherein the acid condition is formed by a heavy water solution which deuteration acid is added in. The total-deuteration tert butyl alcohol preparation method disclosed by the invention has the advantages of moderate reaction condition, high reaction yield, ability in obtaining abundant total-deuteration tert butyl alcohol and simpleness in technological operation.
Owner:上海枢炬化工有限公司
Method for passivating tin oxide/perovskite interface layer of perovskite solar cell by magnesium iodide
PendingCN114709339AImprove conductivityIncrease currentSolid-state devicesSemiconductor/solid-state device manufacturingPerovskite solar cellPhysical chemistry
The invention relates to the technical field of perovskite photovoltaics, and discloses a method for passivating a tin oxide / perovskite interface layer of a perovskite solar cell through magnesium iodide, and the surface of SnO2 is spin-coated with a magnesium iodide ethanol solution prepared in advance. After optimization, a magnesium iodide ethanol solution with the concentration of 1 mg / mL is subjected to spin coating through the procedure of 3000 rpm for 20 s, and the optimal magnesium iodide film thickness is obtained. The efficiency of a perovskite solar cell device manufactured under the thickness is improved, and due to the characteristic of high conductivity of magnesium iodide, the improvement of current is remarkably influenced. And magnesium iodide has a certain inhibition effect on defects at the interface, and iodide ions can reduce the stability problem caused by loss of iodide ions in the perovskite layer.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Unsymmetrical β-diimine monovalent magnesium compound and its preparation method and application
InactiveCN108191891BHigh catalytic activitySimple structureOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsAlkaline earth metalPtru catalyst
The invention discloses an unsymmetrical β-diimine monovalent magnesium compound as well as its preparation method and application. Its preparation method is as follows: first condense acetylacetone with different kinds of aromatic amines respectively to generate asymmetric β-diimine ligands, then react it with an equal amount of methylmagnesium iodide to generate magnesium iodide, and finally use excess The sodium reduction of unsymmetrical β-diimine monovalent magnesium compound. The asymmetric β-diimine monovalent magnesium compound prepared by the invention has a remarkable catalytic effect in the hydroboration reaction of alkynes, the reaction conditions are mild, the reaction speed is fast, the yield can reach more than 90%, and the catalyst has high activity. , which is the first case of using an alkaline earth metal compound to catalyze this type of reaction, which has good practicability.
Owner:NANJING FORESTRY UNIV
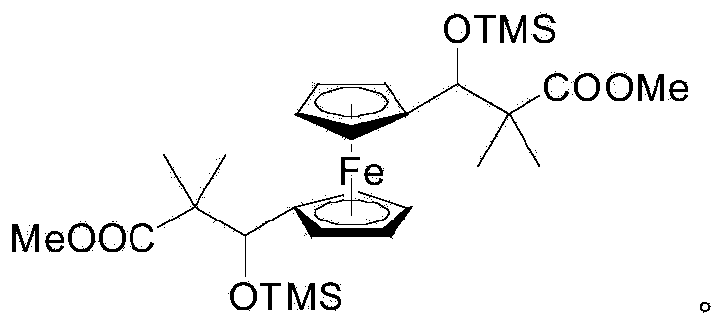
A kind of bismethylsiloxy tricarbonate ferrocene monomer and preparation method thereof
The invention relates to a bis(methylsiloxy)ferrocene tricarbon ester monomer and a preparation method thereof. The chemical structural formula of the bis(methylsiloxy)ferrocene tricarbon ester monomer is shown in a formula I as described in the specification. The preparation method for the monomer comprises a step of reacting 1,1'-ferrocenedialdehyde with 1-methoxy-1-(trimethylsiloxy)-2-methyl-1-propylene under the catalysis of a catalyst--magnesium iodide diethyl ether complex. The bis(methylsiloxy)ferrocene tricarbon ester monomer prepared in the invention can be used for preparation of a conductive material.
Owner:SUZHOU CINC TEXTILE TECH RP
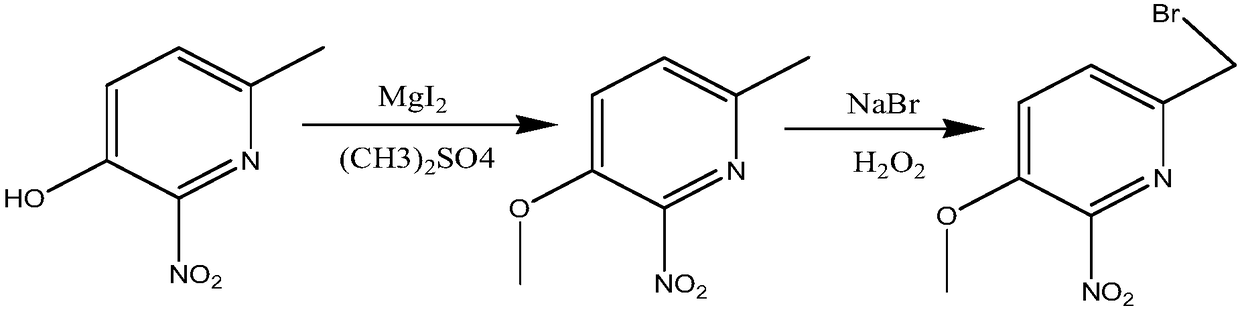
Method for synthesizing 6-bromomethyl-3-methoxy-2-nitropyridine
The invention discloses a method for synthesizing 6-bromomethyl-3-methoxy-2-nitropyridine. The method comprises the synthesizing steps: firstly, mixing 6-methyl-3-hydroxyl-2-nitropyridine, magnesium iodide and water, dropwise adding dimethyl sulfate, carrying out a reaction for 24 hours with heating, carrying out extraction with ethyl acetate, and boiling off the ethyl acetate, so as to obtain 6-methyl-3-methoxyl-2-nitropyridine; and adding carbon tetrachloride and sodium bromide, carrying out stirring for dissolving, dropwise adding hydrogen peroxide, carrying out a reaction with heating, boiling off the carbon tetrachloride, adding 200ml of water, carrying out extraction with ethyl acetate, boiling off the ethyl acetate so as to obtain crude 6-bromomethyl-3-methoxyl-2-nitropyridine, carrying out column chromatography separation, and carrying out recrystallization with acetonitrile water, thereby obtaining the 6-bromomethyl-3-methoxy-2-nitropyridine. According to the method, the yieldis 75%.
Owner:CHANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com