A kind of unsymmetrical β-diimine monovalent magnesium complex and its preparation method and application in nitrile hydroboration
A diimine and asymmetric technology, applied in the field of asymmetric beta-diimine monovalent magnesium complexes and their preparation, can solve problems such as yet, and achieve the effects of fast reaction speed, good selectivity and non-toxic catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
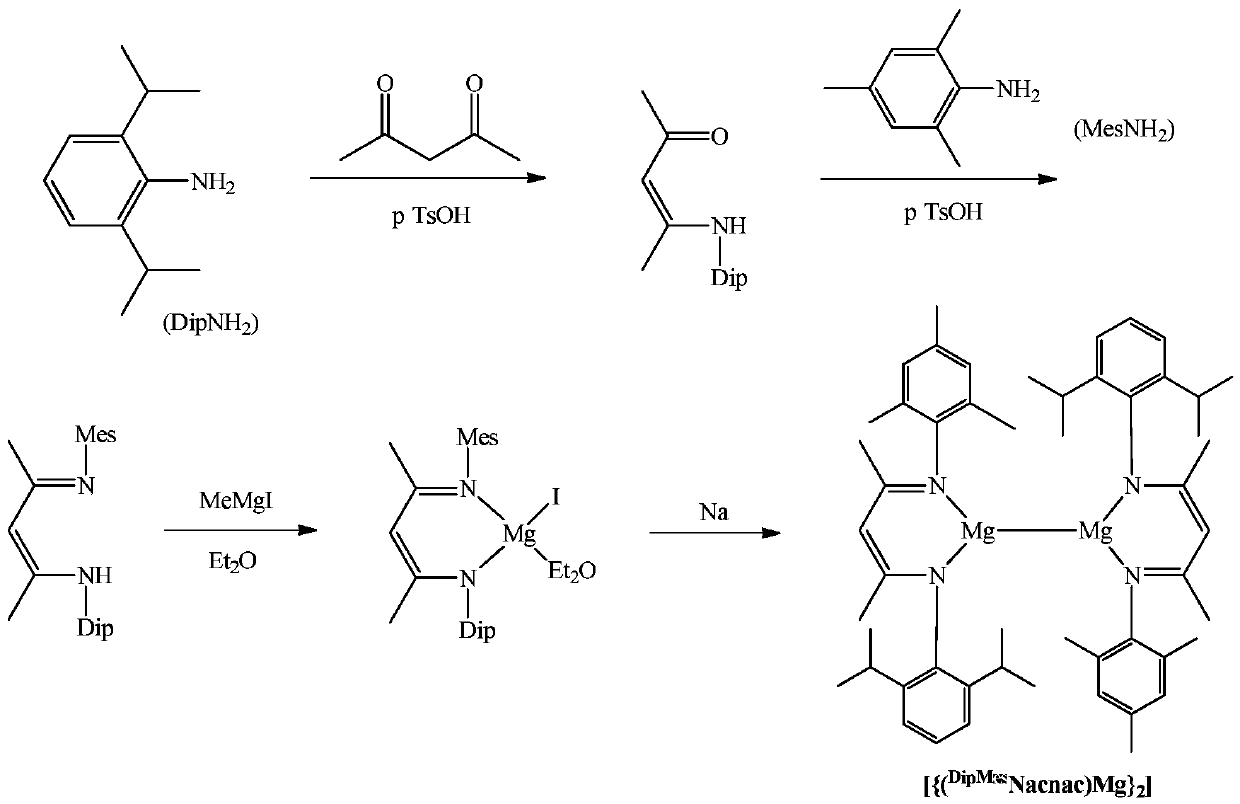
[0033] (1) Asymmetric β-diimine ligand ( DipMes Preparation of Nacnac(H)
[0034] Under argon protection, add 120mL of toluene to a 250mL round bottom flask, then add 147mmol of 2,6-diisopropylaniline, 147mmol of acetylacetone and 1.8mmol of p-toluenesulfonic acid. Reflux for 9 hours, after the reaction is completed, pump dry, then add 147mmol of 2,4,6-trimethylaniline and 147mmol p-toluenesulfonic acid in 120mL of toluene solvent, reflux at 160°C for 24h under the Dean-Stark apparatus, pump dry, use Dichloromethane and saturated NaHCO 3 Extracted three times, the organic phase was extracted with anhydrous MgSO 4 After drying, the obtained solid is exactly the asymmetric β-diimine ligand ( DipMes NacnacH), the yield was 64%. NMR data of the product: 1 H NMR (CDCl 3 ,600MHz):δ12.27(s,1H,NH),7.13(s,3H,Ar-H),6.89(s,2H,Ar-H),4.89(s,1H,=CH),3.08(sept , 3 J HH =6.6Hz,2H,CH(CH 3 ) 2 ),2.28(s,3H,CH 3 ),2.16(s,6H,CH 3 ),1.73(s,3H,NCCH 3 ),1.72(s,3H,NCCH 3 ),1.24(d, 3 J ...
Embodiment 2
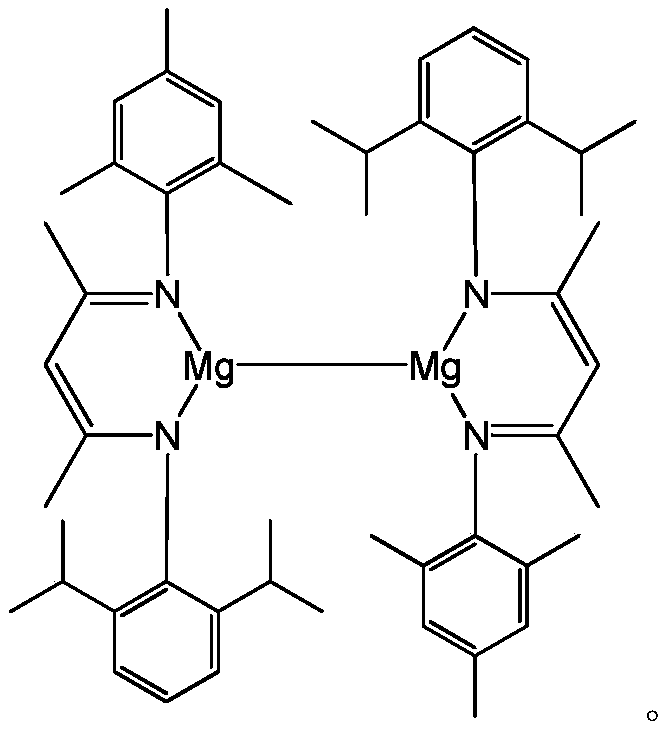
[0037] Example 2: [{( DipMes Nacnac)Mg} 2 ] Catalyzed hydroboration reaction of benzonitrile and pinacol borane
[0038] Under anhydrous and oxygen-free conditions, in the glove box, [{( DipMes Nacnac)Mg} 2 ]0.006mmol was added to about 0.5mL of C 6 D. 6 Then add 1.2mmol of pinacol borane with a pipette gun and mix well, and finally add 0.6mmol of benzonitrile, react at 60°C for 8h, and measure the NMR. Calculated 1 H spectrum yield was 98%. NMR data of the product: 1 H NMR (C 6 D. 6 ,600MHz):7.58(m,2H,Ar-H),7.25(m,2H,Ar-H),7.11(m,1H,Ar-H),4.61(s,2H,Ar-CH 2 ),1.03(s,24H,C(CH 3 ) 2 ).
Embodiment 3
[0039] Example 3: [{( DipMes Nacnac)Mg} 2 ] Catalytic hydroboration of 4-methoxybenzonitrile and pinacol borane
[0040] Under anhydrous and oxygen-free conditions, in the glove box, [{( DipMes Nacnac)Mg} 2 ]0.006mmol was added to about 0.5mL of C 6 D. 6 Then add 1.2mmol of pinacol borane with a pipette gun and mix well, and finally add 0.6mmol of 4-methoxybenzonitrile, react at 60°C for 24h, and measure NMR. Calculated 1 H spectrum yield was 83%. NMR data of the product: 1 HNMR (C 6 D. 6 ,600MHz):7.56(d, 3 J HH =8.4Hz, 2H, Ar-H), 6.87(d, 3 J HH =8.4Hz,2H,Ar-H),4.58(s,2H,Ar-CH 2 ),3.34(s,3H,OCH 3 ),1.05(s,24H,C(CH 3 ) 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com