Unsymmetrical β-diimine monovalent magnesium compound and its preparation method and application
A technology of magnesium compounds and diimine, which is applied in the preparation of imino compounds, magnesium organic compounds, chemical instruments and methods, etc., can solve the problems such as the lack of alkaline earth metal compounds, and achieve simple structure, easy synthesis and high catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of asymmetric β-diimine ligands
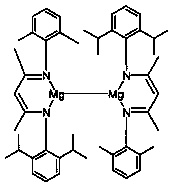
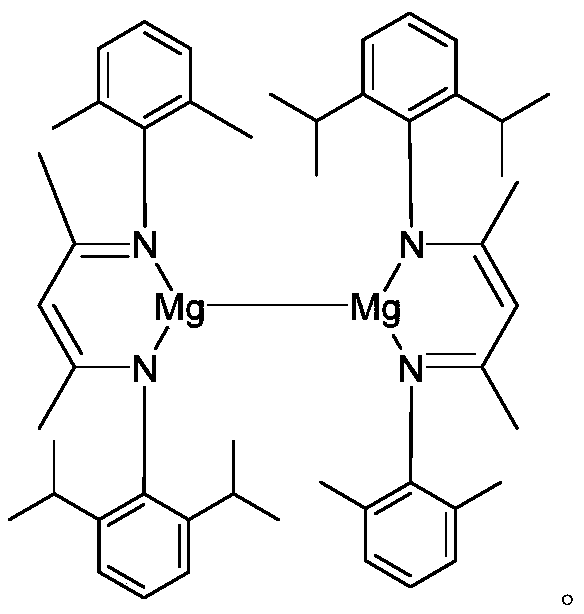
[0034] Under argon protection, add 140mL of toluene to a 250mL round bottom flask, then add 132mmol of 2,6-diisopropylaniline, 132mmol of acetylacetone and 1.6mmol of p-toluenesulfonic acid. Reflux for 8 hours, after the reaction is completed, pump dry, then add 132 mmol of 2,6-dimethylaniline and 132 mmol of p-toluenesulfonic acid, reflux for 21 hours at 150°C in a Dean-Stark apparatus in 130 mL of toluene solvent, pump dry, and use two Methyl chloride and saturated NaHCO 3 Extracted three times, the organic phase was extracted with anhydrous MgSO 4 After drying and drying, the obtained solid is an asymmetric β-diimine ligand, referred to as DipXyl NacnacH, 71% yield.
[0035] Characterize the product, NMR data: 1 H NMR (CDCl 3 ,600MHz):δ12.27(s,1H,NH),7.13(s,3H,Ar-H),6.89(s,2H,Ar-H),4.89(s,1H,=CH),3.08(sept , 3 J HH =6.6Hz,2H,CH(CH 3 ) 2 ),2.28(s,3H,CH 3 ),2.16(s,6H,CH 3 ),1.73(s,3H,NCCH 3 ),1.72(s,3H,NCCH...
Embodiment 2
[0039] Example 2: [{( DipXyl Nacnac)Mg} 2 ] Catalyzed Hydroboration of Phenylacetylene and Pinacacol Borane
[0040] Under anhydrous and oxygen-free conditions, in the glove box, [{( DipXyl Nacnac)Mg} 2 ]0.006mmol was added to about 0.5mL of toluene-d 8 Then add 0.18 mmol of pinacol borane with a pipette gun and mix evenly, and finally add 0.12 mmol of phenylacetylene, react at 110°C for 6 hours, and measure NMR. Calculated 1 H spectrum yield was 99%.
[0041] NMR data of the product: 1 H NMR (600MHz, tol-d 8 ):δ7.63(d, 3 J HH =18.6Hz,2H),7.30(d, 3 J HH =7.2Hz,2H),7.06(d, 3 J HH =7.2Hz,2H),6.35(d, 3 J HH =18.6Hz,2H),1.16(s,12H). 13 C{ 1 H}NMR (151MHz, tol-d 8 ): δ149.7, 137.1, 128.5, 128.3, 127.0, 116.2, 82.7, 24.5. 11 B NMR (193MHz, tol-d 8 ): δ30.37.
Embodiment 3
[0042] Example 3: [{( DipXyl Nacnac)Mg} 2 ]Catalyzed hydroboration reaction of p-methoxyphenylacetylene with pinacol borane
[0043] Under anhydrous and oxygen-free conditions, in the glove box, [{( DipXyl Nacnac)Mg} 2 ]0.006mmol was added to about 0.5mL of toluene-d 8 Then add 0.18mmol of pinacol borane with a pipette gun and mix evenly, and finally add 0.12mmol of p-methoxyphenylacetylene, react at 110°C for 10h, and measure NMR. Calculated 1 H spectrum yield was 98%.
[0044] NMR data of the product: 1 H NMR (600MHz, tol-d 8 ):δ7.62(d, 3 J HH =18.6Hz,2H),7.23(d, 3 J HH =8.4Hz,2H),6.60(d, 3 J HH =8.4Hz,2H),6.22(d, 3 J HH =18.6Hz,2H),3.26(s,3H).1.16(s,12H). 13 C{ 1 H}NMR (151MHz, tol-d 8 ): δ160.8, 149.8, 137.5, 131.0, 114.25, 83.0, 25.0. 11 BNMR (193MHz, tol-d 8 ): δ31.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com