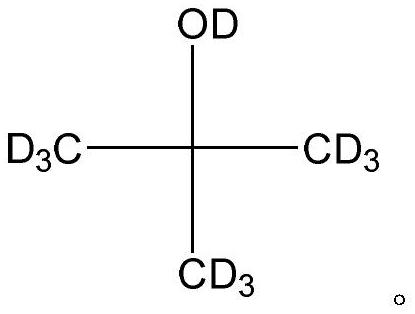
A kind of preparation method of all deuterated tert-butanol
A deuterated tert-butanol and deuterated technology, which is applied in the field of organic synthesis, can solve the problem of no high-abundance per-deuterated tert-butanol synthesis method, is not suitable for large-scale preparation of per-deuterated tert-butanol, and has no practical value. and other problems, to achieve the effect of improving the convenience of operation, improving the conversion rate and product yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
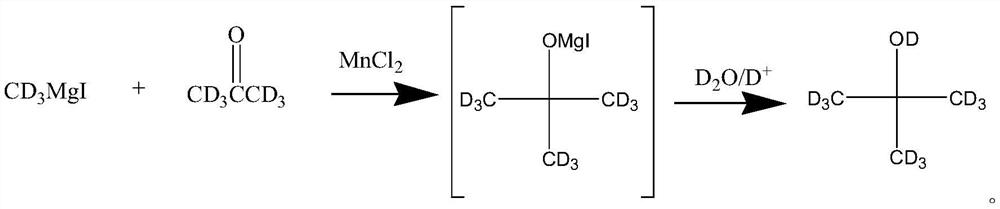
[0019] The inventors of the present application have unexpectedly found that when deuterium methylmagnesium iodide is used as the Grignard reagent and anhydrous manganese chloride is used as the catalyst, a very ideal yield can be obtained, and the obtained all-deuterated tert-butanol High abundance. For this reason, the present invention provides a kind of preparation method of full deuterium tert-butanol, it comprises the steps: make deuterium methylmagnesium iodide and deuterium acetone in anhydrous tetrahydrofuran, in the presence of anhydrous manganese chloride Carry out Grignard reaction, and then carry out hydrolysis to obtain all deuterated tert-butanol.
[0020] Concrete reaction process is as follows;
[0021]
Embodiment 1
[0025] This example provides a method for preparing all-deuterated tert-butanol, specifically: in a 2000mL three-necked flask equipped with a thermometer and a condenser tube, add 185g of deuteromethylmagnesium iodide, 1000mL of anhydrous tetrahydrofuran, and 8g of anhydrous chlorine Manganese chloride, then dropwise add 64g deuterated acetone, react at 10±2°C for 4-6 hours, then add heavy aqueous solution dissolved in deuterium chloride to the above-mentioned flask to quench the reaction, and obtain all deuterated tert-butanol The reaction solution, after rectification, can obtain the high-abundance perdeuterated tert-butanol product: 67g, the yield is 80%, the detection of the perdeuterated tert-butanol product, the GC purity is 99.9%, and the isotope abundance detected by mass spectrometry is 99.8 %(atom %D). Elemental analysis theoretical value: C 57.07%, D23.92%, O19.00%; measured value: C 57.05%, D23.91%, O19.02%.
Embodiment 2
[0027] This example provides a method for preparing all-deuterated tert-butanol, specifically: in a 2000mL three-necked flask equipped with a thermometer and a condenser tube, add 185g of deuteromethylmagnesium iodide, 1000mL of anhydrous tetrahydrofuran, and 8g of anhydrous chlorine Manganese chloride, then dropwise add 64g deuterated acetone, react at -10±2°C for 4-6 hours, then add heavy aqueous solution dissolved in deuterated sulfuric acid to the above-mentioned flask to quench the reaction, and obtain all deuterated tert-butyl Alcohol reaction solution, through rectification, obtains the high-abundance per-deuterated tert-butanol product: 69g, yield 82%, detects the per-deuterated-tert-butanol product, GC purity is 99.9%, and the isotope abundance detected by mass spectrometry is 99.7% (atom% D). Elemental analysis theoretical value: C 57.07%, D23.92%, O19.00%; measured value: C 57.06%, D23.91%, O19.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com