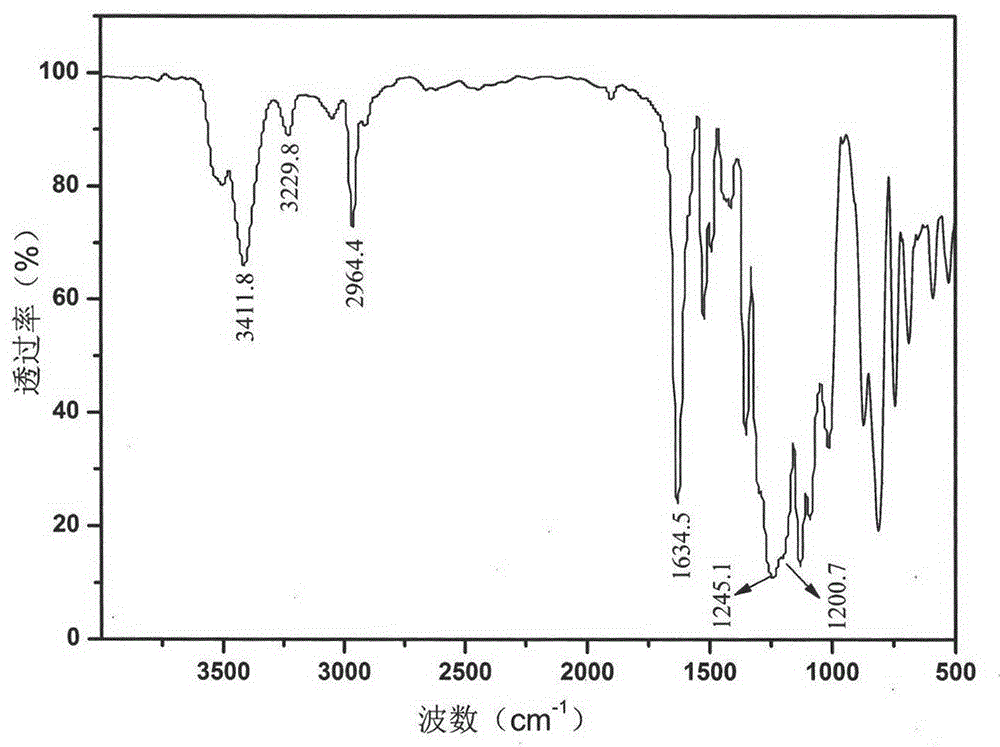
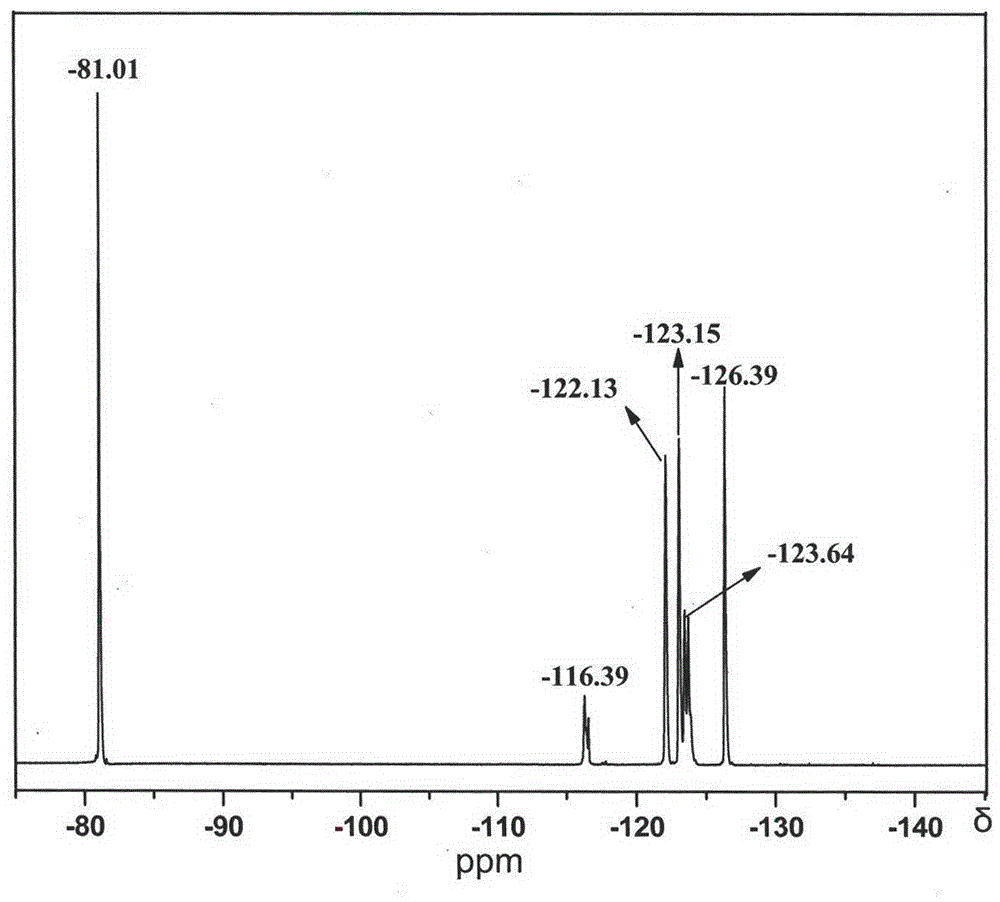
Fluorine-containing organosilicone monomer and preparation method thereof
A technology of organosilicon and monomers, which is applied in the direction of organic chemistry, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve the problem that short fluoroalkyl groups do not have crystallinity and cannot be arranged in liquid crystal structure, short fluorine Problems such as application limitation of alkylation materials, reduction of water and oil repellency performance, etc., to achieve the effect of environmentally friendly surface activity, high fluorine content, and low surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a four-necked flask equipped with a reflux condenser, mechanical stirring and heating device, put 400 grams of 3-tridecafluorohexylpropene and 500 grams of tridecafluoro-1-iodohexane, and heat the mixture to 88 ° C, under nitrogen protection Add 10 grams of initiator azobisisobutyronitrile, and stir the reaction mixture at 88°C for 48 hours. During this period, add azobisisobutyronitrile three times every 12 hours, and add 2 grams of azobisisobutyronitrile each time. Nitrile. After the reaction is over, stop the reaction, and rectify (the temperature is 150°C, the vacuum degree is 2mmHg) to obtain a fraction at 106-107°C, which is the product 1,3-bis(tridecafluorohexyl)-2-iodopropane, with a yield of 97.0 %.
[0036]In a three-necked flask equipped with a reflux condenser, a thermometer, and a constant pressure dropping funnel, add 2.9 grams of magnesium chips and 50 grams of anhydrous tetrahydrofuran, introduce nitrogen, start stirring with a magnetic stirrer, and ...
Embodiment 2
[0048] 1,3-bis(tridecafluorohexyl)-2-iodopropane was prepared according to Example 1.
[0049] In a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, add 3.1 grams of magnesium chips and 55 grams of anhydrous ether, introduce nitrogen, start stirring with a magnetic stirrer, and heat the system to 30 ° C with a water bath, 69.2 g of 1,3-bis(tridecafluorohexyl)-2-iodopropane was added dropwise through a constant pressure dropping funnel for a total of 1 hour and 15 minutes. After the addition, the reaction was continued for 6 hours to obtain a suspension of 1,3-bis(tridecafluorohexyl)-2-propylmagnesium iodide.
[0050] The above 1,3-bis(tridecafluorohexyl)-2-propylmagnesium iodide suspension was cooled to -3°C and set aside.
[0051] In a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, add 12.9 grams of tetrachlorosilane and 55 grams of anhydrous ether, introdu...
Embodiment 3
[0060] 1,3-bis(tridecafluorohexyl)-2-iodopropane was prepared according to Example 1.
[0061] In a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, add 3.5 grams of magnesium chips and 60 grams of anhydrous ether, introduce nitrogen, start stirring with a magnetic stirrer, and heat the system to 30 ° C with a water bath, 69.5 g of 1,3-bis(tridecafluorohexyl)-2-iodopropane was added dropwise through a constant-pressure dropping funnel for a total of 1 hour. After the addition, the reaction was continued for 6 hours to obtain a suspension of 1,3-bis(tridecafluorohexyl)-2-propylmagnesium iodide.
[0062] The above 1,3-bis(tridecafluorohexyl)-2-propylmagnesium iodide suspension was cooled to -5°C and set aside.
[0063] In a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, add 7.2 grams of tetrachlorosilane and 48 grams of anhydrous ether, introduce nitrogen, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com