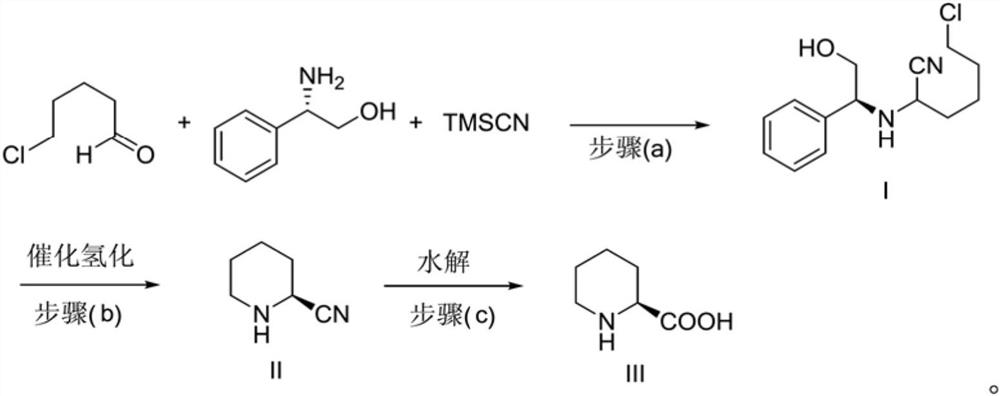
Preparation method of caine drug intermediate (S)-2-piperidinecarboxylic acid
A technology of piperidinecarboxylic acid and cyanopiperidine is applied in the field of preparation of caine-type drug intermediate-2-piperidinecarboxylic acid, which can solve the problems such as difficulty in obtaining starting materials, high production cost, complicated steps, etc. Mild conditions, improved safety, and good reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 compound I
[0045] Add 1g (8.3mmol) of 5-chlorovaleraldehyde, 1.25g (9.13mmol) of L-phenylglycinol, and 1g (10mmol) of trimethylsilylcyanide into the reaction flask, and then add 115mg (0.415mmol) of magnesium diiodide , Stir the reaction at room temperature (25° C.) for 4 h. TLC monitors the reaction process. After the reaction is completed, the reaction solution is purified by silica gel column chromatography. The eluent is sherwood oil: ethyl acetate (volume ratio: 5: 1), and after concentration, 209 mg of light yellow oily product I is obtained. The yield is 95%. HPLC area normalization method detection purity is 98% [chromatographic condition: chromatographic column Kromasil C 18Column (4.6mm×250mm, 5μm), mobile phase: acetonitrile-water (volume ratio 70:30), detection wavelength: 254nm, column temperature: 25°C, flow rate: 1.0mL·min -1 ]. Optical purity 98% [chromatographic conditions: chiral chromatographic column CHIRALPAK AD-H...
Embodiment 2
[0046] The synthesis of embodiment 2 compound I
[0047] Add 1g (8.3mmol) of 5-chlorovaleraldehyde, 1.25g (9.13mmol) of L-phenylglycinol, and 1g (10mmol) of trimethylsilylcyanide into the reaction flask, and then add 76mg (0.415mmol) of magnesium dibromide , Stir the reaction at room temperature (25° C.) for 6 h. TLC monitors the reaction process. After the reaction is completed, the reaction solution is purified by silica gel column chromatography. The eluent is sherwood oil: ethyl acetate (volume ratio: 5: 1). After concentration, 171 mg of the light yellow oily product I is obtained. The yield is 78%. HPLC area normalization method detection purity is 98% [chromatographic condition: chromatographic column Kromasil C 18 Column (4.6mm×250mm, 5μm), mobile phase: acetonitrile-water (volume ratio 70:30), detection wavelength: 254nm, column temperature: 25°C, flow rate: 1.0mL·min -1 ]. Optical purity 98% [chromatographic conditions: chiral chromatographic column CHIRALPAK AD-...
Embodiment 3
[0048] The synthesis of embodiment 3 compound I
[0049] Add 1g (8.3mmol) of 5-chlorovaleraldehyde, 1.25g (9.13mmol) of L-phenylglycinol, and 1.2g (12mmol) of trimethylsilylcyanide into the reaction flask, and then add 186mg (0.8mmol) of magnesium perchlorate ), stirred at room temperature (25° C.) for 8 h. TLC monitors the reaction process. After the reaction is completed, the reaction solution is purified by silica gel column chromatography. The eluent is sherwood oil: ethyl acetate (volume ratio: 5: 1), and after concentration, 158 mg of light yellow oily product I is obtained. 72%. HPLC area normalization method detection purity is 98% [chromatographic condition: chromatographic column Kromasil C 18 Column (4.6mm×250mm, 5μm), mobile phase: acetonitrile-water (volume ratio 70:30), detection wavelength: 254nm, column temperature: 25°C, flow rate: 1.0mL·min -1 ]. Optical purity 97% [chromatographic conditions: chiral chromatographic column CHIRALPAK AD-H (250mm×4.6mm, 5μm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com