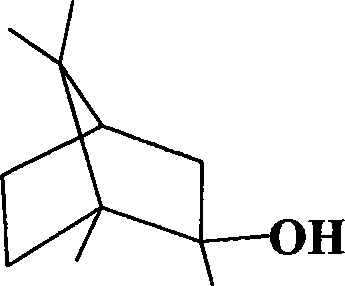
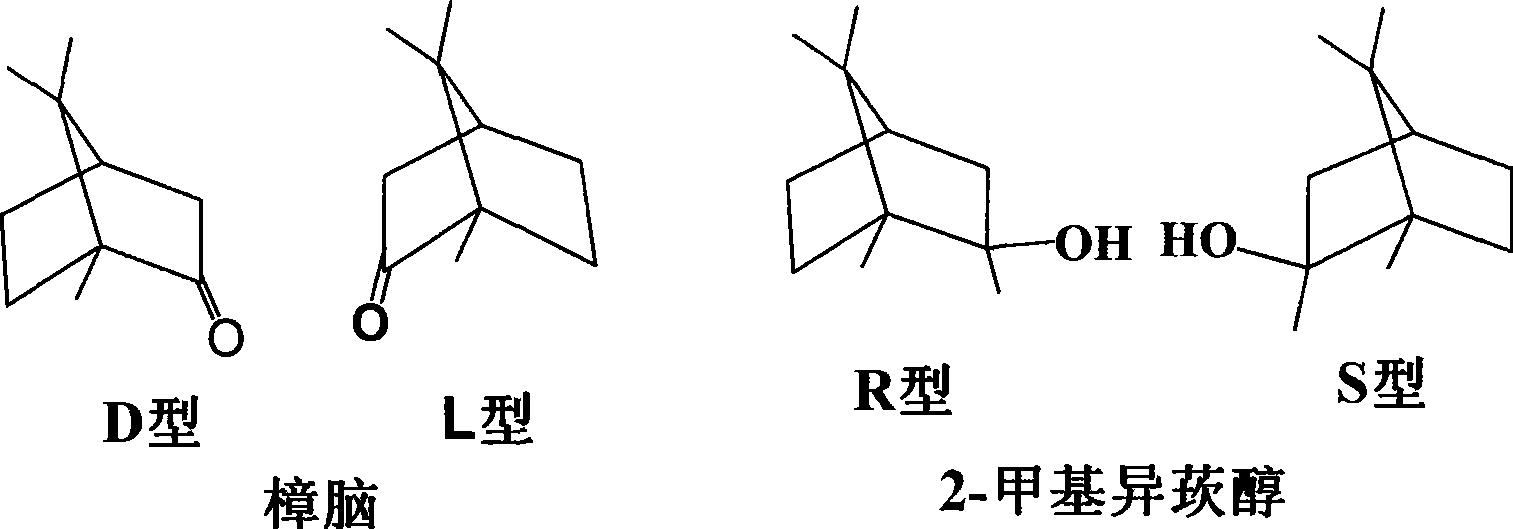
Method for synthesizing 2-methylisoborneol
A technology of methyl isoborneol and mixed solution, which is applied in the field of synthesizing smell and taste substance-2-methylisoborneol, which can solve the problems of high cost, many influencing factors, and long cultivation period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The removal test of the product oxime after the reaction of embodiment 1 to camphor and hydroxylamine hydrochloride.
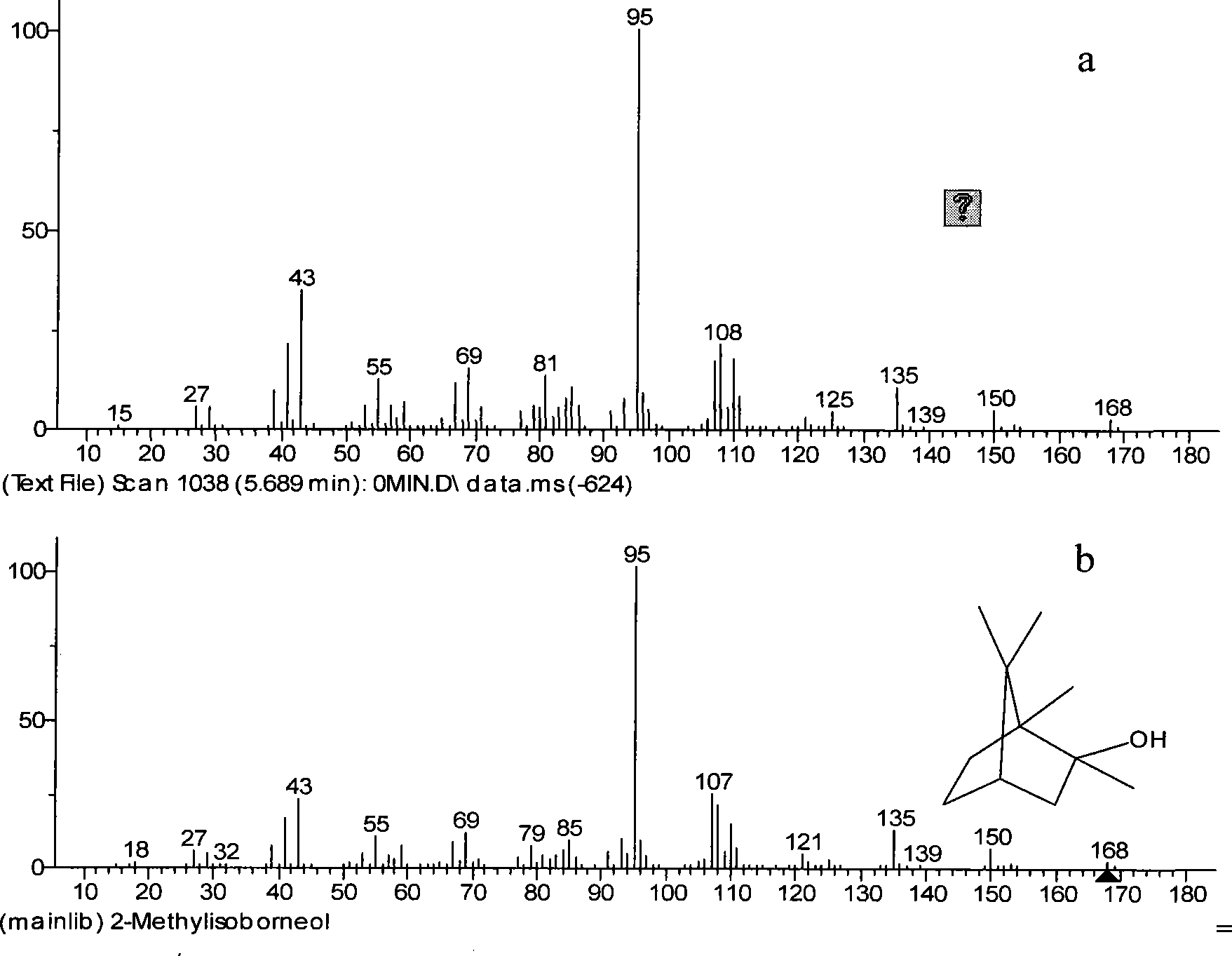
[0042] Because the product oxime after the reaction of camphor and hydroxylamine hydrochloride is acidic, it is diluted with distilled water and then washed with 2 mol / liter of sodium hydroxide solution, extracted twice with normal hexane, and the residual solution after extraction Figure 4c combined with n-hexane Figure 4d Carry out gas chromatographic measurement, find that greater than 98% of 2-methylisoborneol and less than 50% of oxime are in the extractant n-hexane.
Embodiment 2
[0047] In order to reduce the content of camphor in the crude product, it is necessary to find a reagent that can selectively react with camphor to generate a large number of derivatives. The resulting derivative needs to be more easily separated from 2-methylisoborneol than camphor itself. But camphor itself is inert, and many reagents are not very effective. And contriver has chosen hydroxylamine hydrochloride to react with camphor under microboiling condition, finds by gas chromatography to reaction on-line monitoring, when reaction times is 2 hours, remaining 19% camphor (see Figure 4b ); When the reaction time was 7 hours, camphor had been completely converted into the derivative of oxime (see Figure 4c ). The determination gas chromatography result of final product sees Figure 4e shown. Therefore the present invention selects 8 hours as preferred reflux reaction time.
[0048] The above crude product was separated and purified by silica gel column, with dichlorom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com