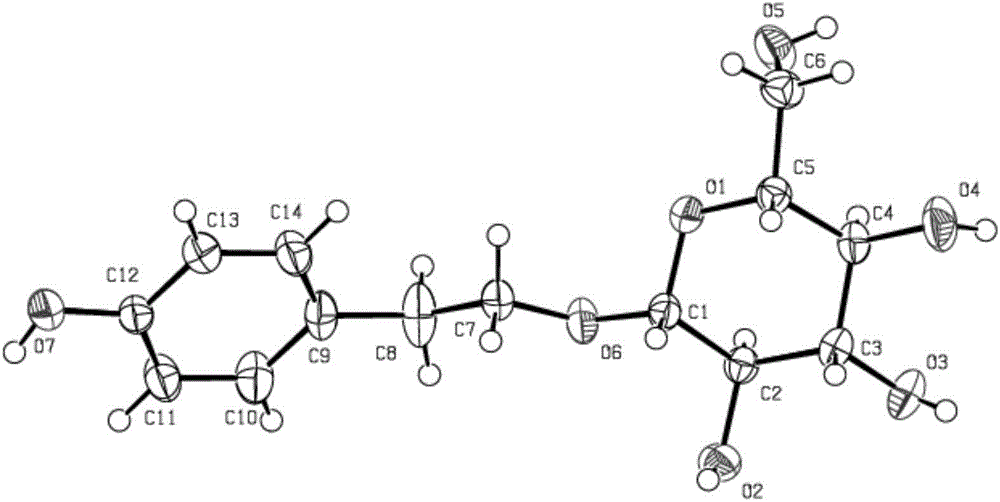
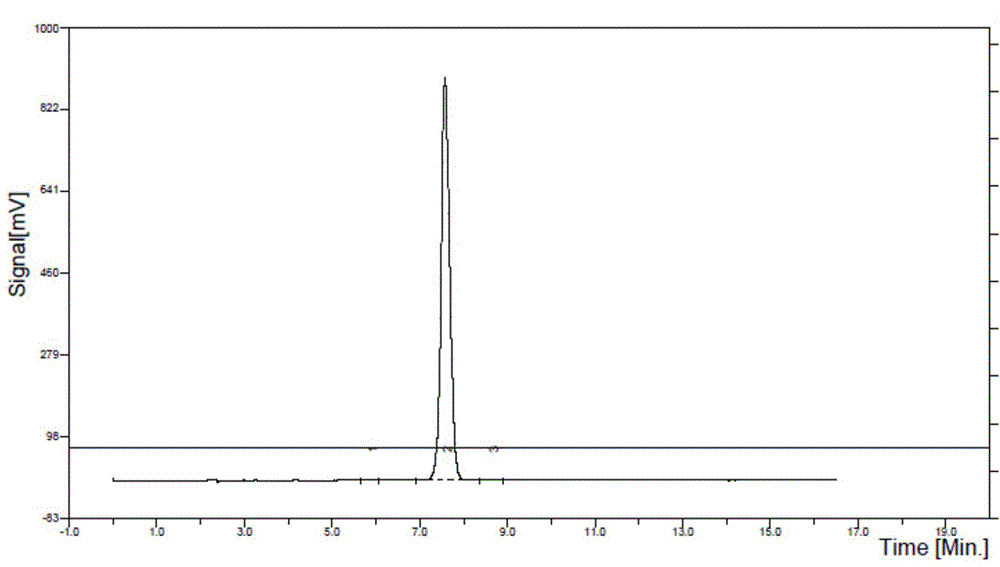
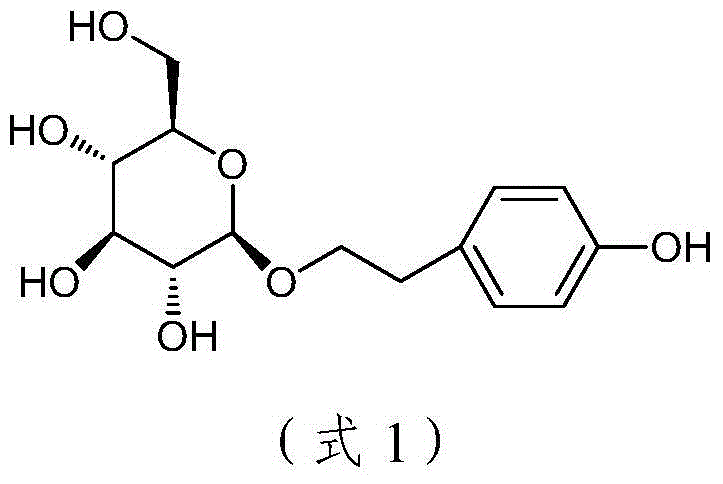
Method for catalytic synthesis of salidroside
A salidroside and synthetic method technology, applied in chemical instruments and methods, organic chemistry, bulk chemical production, etc., can solve the problems of low yield and high production cost, and achieve high product yield, low cost, and catalytic The effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Synthesis of intermediate 4-benzyloxyphenethyl alcohol:
[0045] Dissolve 600g (4.348mol) of 4-hydroxyphenethyl alcohol in 1 liter of DMF. After dissolving, add 178g (4.45mol) of sodium hydroxide and stir for half an hour. Then add 481g (4.829mol) of benzyl chloride. TLC monitors that there is no raw material benzyl chloride. For post-treatment, it was directly poured into ice water, stirred for half an hour, filtered, washed with water, and dried to obtain 842 g of white solid, with a yield of 85%.
[0046] (2) Preparation of 2-(4-benzyloxyphenyl)ethyl-(2,3,4,6-O-tetra-acetyl)-β-D-glucopyranoside:
[0047] Add 273g (0.70mol) of peracetyl glucose, 191.5g (0.84mol) of 4-benzyloxy phenethyl alcohol, 29g (0.21mol) of zinc chloride and 13.6g (0.14mol) of magnesium chloride directly into 1.5L of chloroform Stir, react at 60°C for 10 hours and then desolvate to obtain off-white solid 2-(4-benzyloxyphenyl)ethyl-(2,3,4,6-O-tetra-acetyl)-β-D-pyran Glucoside 289g (0.51mol), the yi...
Embodiment 2
[0065] The preparation of 2-(4-benzyloxyphenyl) ethyl-(2,3,4,6-O-tetra-acetyl)-β-D-glucopyranoside: 200g (0.513 mol), 196g (0.86mol) of 4-benzyloxy phenethyl alcohol, 129g (0.97mol) of aluminum trichloride, 50g (0.513mol) of magnesium chloride, directly added to 1.2L of chloroform and stirred, reacted at 60℃ for 10h, and then dissolved , To obtain 200 g (0.36 mol) of off-white solid, with a yield of 70%.
Embodiment 3
[0067] Preparation of 2-(4-benzyloxyphenyl) ethyl-(2,3,4,6-O-tetra-acetyl)-β-D-glucopyranoside: 279 g (0.717 mol), 196g (0.86mol) of 4-benzyloxyphenethyl alcohol, 186g (0.717mol) of tin chloride and 44g (0.717mol) of magnesium fluoride are directly added to 1.6L of dichloroethane and stirred, and reacted at 60°C for 15h After desolvation, 281 g (0.49 mol) of off-white solid was obtained with a yield of 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com