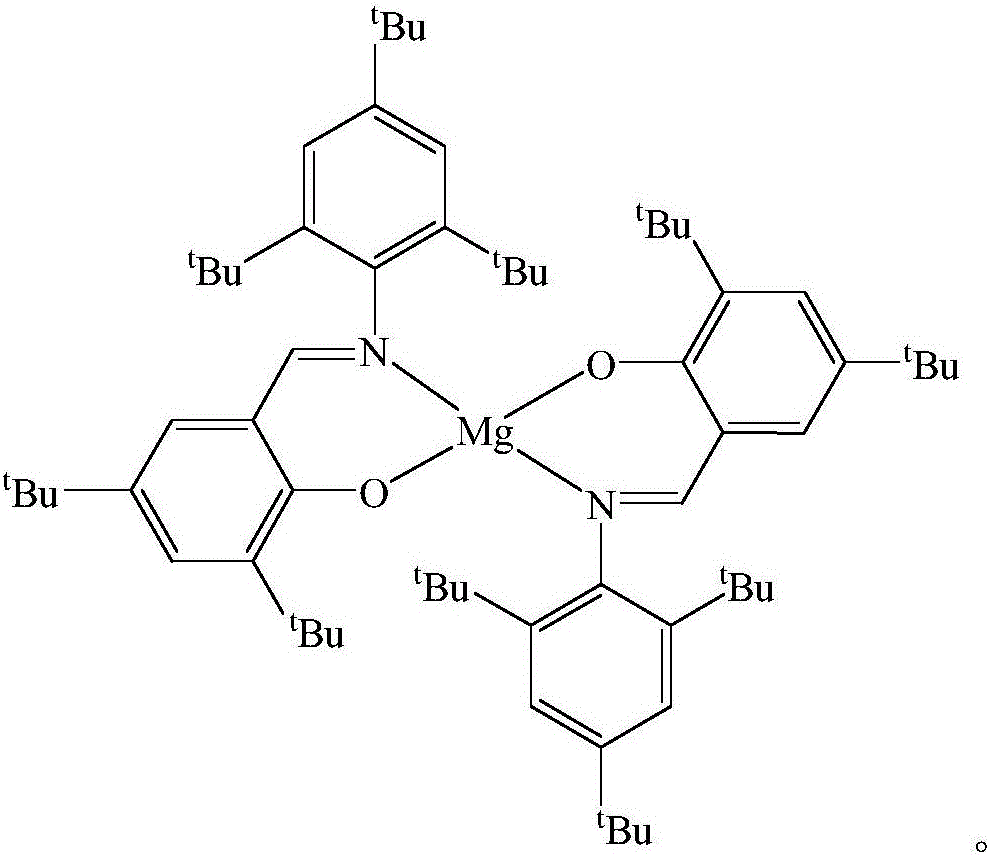
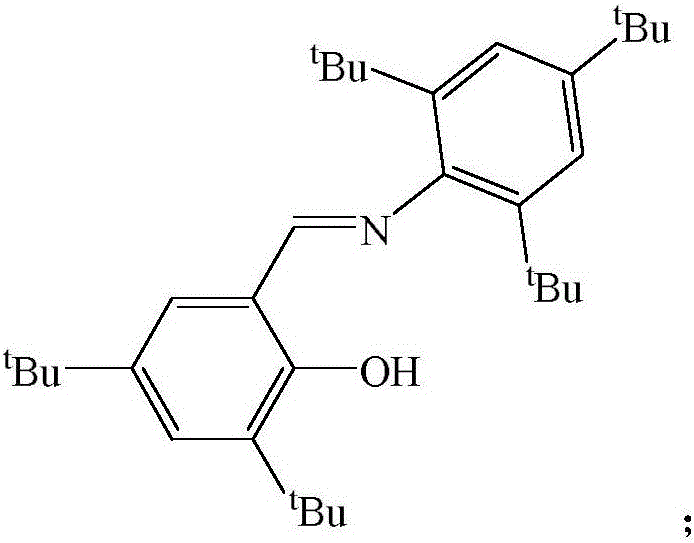
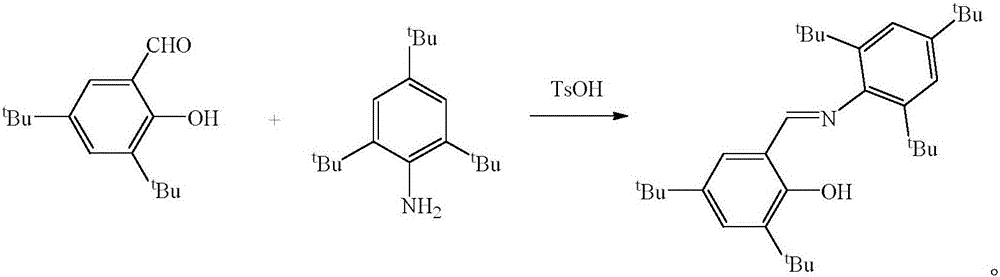
Schiff base magnesium organometallic compound, and preparation method and application thereof
A metal compound, Schiff base magnesium technology, applied in the field of metal-organic compound preparation, can solve the problem of no Schiff base magnesium metal compound sodium-magnesium bimetallic synthesis and application, etc., to achieve simple and easy operation of the reaction process, easy product and good quality less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base ligand is as follows:
[0037] 10.04mmol of 2,4,6-tri-tert-butylaniline, 10.04mmol of 3,5-di-tert-butyl salicylaldehyde, 1.67mmol of p-toluenesulfonic acid and 100mL of ethanol were added to a 100mL round-bottom flask, and the reaction was refluxed for 48h. A large number of light yellow crystals precipitated at -30°C, filtered, the mass was 4.32g, which was 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base, the yield was 90% . M.p.178-180°C.
[0038] NMR spectrum: 1 H NMR (600MHz, 298K, C 6 D. 6 ): δ1.26(s, 9H, C(CH 3 ) 3 ), 1.38(s, 9H, C(CH 3 ) 3 ), 1.39(s, 18H, C(CH 3 ) 3 ), 1.65(s, 9H, C(CH 3 ) 3 ), 7.03 (d, J H-H =2.4Hz, 1H, Ar-H), 7.56(s, 2H, Ar-H), 7.63(d, J H-H =2.4Hz, 1H, Ar-H), 7.92(s, 1H, CH=N), 14.14(s, 1H, OH) ppm. 13 C{ 1 H}NMR (151MHz, C 6 D. 6 ): δ29.8, 31.6, 31.8, 32.4, 34.3, 35.0, 35.5, 36.1, 118.1, 122.2, 126.8, 128.4, 128.6, 137.8, 140.9, 141.0, 146.0, 148...
Embodiment 2
[0040] The preparation of 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base ligand is as follows:
[0041] 10.0mmol of 2,4,6-tri-tert-butylaniline, 10.0mmol of 3,5-di-tert-butyl salicylaldehyde, 2.5mmol of p-toluenesulfonic acid and 100mL of ethanol were added to a 100mL round-bottomed flask, and the reaction was refluxed for 24h. A large number of light yellow crystals were precipitated at -10°C. After filtration, the mass was 4.2 g, which was 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base, and the yield was 89%. . M.p.178-180°C.
[0042] NMR spectrum: 1 H NMR (600MHz, 298K, C 6 D. 6 ): δ1.26(s, 9H, C(CH 3 ) 3 ), 1.38(s, 9H, C(CH 3 ) 3 ), 1.39(s, 18H, C(CH 3 ) 3 ), 1.65(s, 9H, C(CH 3 ) 3 ), 7.03 (d, J H-H =2.4Hz, 1H, Ar-H), 7.56(s, 2H, Ar-H), 7.63(d, J H-H =2.4Hz, 1H, Ar-H), 7.92(s, 1H, CH=N), 14.14(s, 1H, OH) ppm. 13 C{ 1 H}NMR (151MHz, C 6 D. 6 ): δ29.8, 31.6, 31.8, 32.4, 34.3, 35.0, 35.5, 36.1, 118.1, 122.2, 126.8, 128.4, 128.6, 137.8, 140.9, ...
Embodiment 3
[0044] The preparation of 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base ligand is as follows:
[0045] Add 9.0mmol of 2,4,6-tri-tert-butylaniline, 9.0mmol of 3,5-di-tert-butyl salicylaldehyde, 3mmol of p-toluenesulfonic acid and 100mL of ethanol into a 100mL round-bottomed flask, and react under reflux for 12h. A large number of light yellow crystals were precipitated at 4°C. After filtration, the mass was 3.86 g, which was 2-(2,4,6-tri-tert-butyl)-3,5-di-tert-butyl Schiff base, and the yield was 90%. M.p.178-180°C.
[0046] NMR spectrum: 1 H NMR (600MHz, 298K, C 6 D. 6 ): δ1.26(s, 9H, C(CH 3 ) 3 ), 1.38(s, 9H, C(CH 3 ) 3 ), 1.39(s, 18H, C(CH 3 ) 3 ), 1.65(s, 9H, C(CH 3 ) 3 ), 7.03 (d, J H-H =2.4Hz, 1H, Ar-H), 7.56(s, 2H, Ar-H), 7.63(d, J H-H =2.4Hz, 1H, Ar-H), 7.92(s, 1H, CH=N), 14.14(s, 1H, OH) ppm. 13 C{ 1 H}NMR (151MHz, C 6 D. 6 ): δ29.8, 31.6, 31.8, 32.4, 34.3, 35.0, 35.5, 36.1, 118.1, 122.2, 126.8, 128.4, 128.6, 137.8, 140.9, 141.0, 146.0, 148.5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com