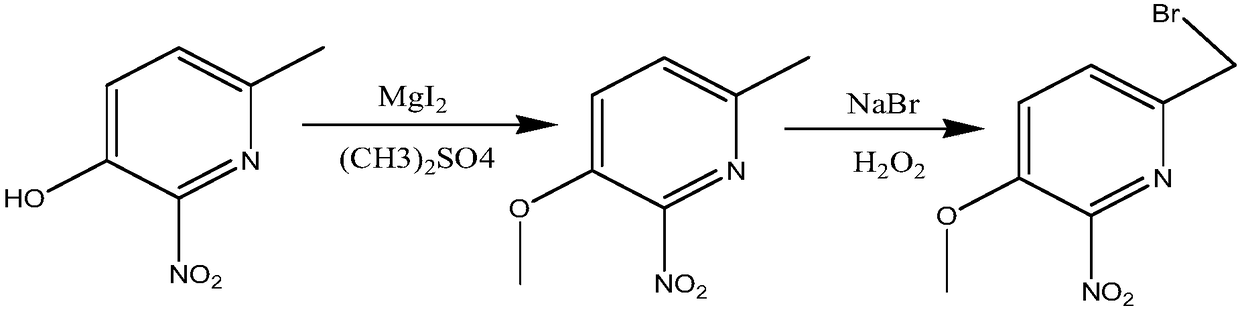
Method for synthesizing 6-bromomethyl-3-methoxy-2-nitropyridine
A technology of nitropyridine and methoxy, applied in the field of synthesizing 6-bromomethyl-3-methoxy-2-nitropyridine, which can solve the problems of difficult reaction temperature and difficult industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Add 15.4g of 6-methyl-3-hydroxy-2-nitropyridine, 16.5g of magnesium iodide and 100g of water into a 250mL four-necked bottle, heat the water bath to 75°C, add dropwise 14.5g of dimethyl sulfate to react 18 hours; add 200mL ethyl acetate for extraction, separate the water layer, evaporate the ethyl acetate, and recrystallize from ethanol to obtain 16.1 g of 6-methyl-3-methoxy-2-nitropyridine. Add 10.1g of 6-methyl-3-methoxy-2-nitropyridine, 10.3g of sodium bromide and 100mL of carbon tetrachloride into a 500mL four-necked bottle, heat to reflux, add 11g of hydrogen peroxide dropwise, and dropwise add Then continue to react for 36 hours; after the reaction, add 100mL of 1M sodium hydroxide solution, cool, add 200mL of ethyl acetate for extraction, and distill off the ethyl acetate to obtain a yellow oil; the yellow oil is separated by alumina column chromatography, and then 75 % acetonitrile aqueous solution to obtain 11.1 g of light yellow 6-bromomethyl-3-methoxy-2-nitro...
example 2
[0020] Add 15.4g of 6-methyl-3-hydroxy-2-nitropyridine, 16.5g of magnesium iodide and 100g of water into a 250mL four-necked bottle, heat the water bath to 75°C, add dropwise 14.5g of dimethyl sulfate to react 20 hours; add 200mL ethyl acetate for extraction, separate the water layer, evaporate the ethyl acetate, and recrystallize from ethanol to obtain 16.1 g of 6-methyl-3-methoxy-2-nitropyridine. Add 10.1g of 6-methyl-3-methoxy-2-nitropyridine, 10.3g of sodium bromide and 100mL of carbon tetrachloride into a 500mL four-necked bottle, heat to reflux, add 11g of hydrogen peroxide dropwise, and dropwise add Then continue to react for 40 hours; after the reaction, add 100mL of 1M sodium hydroxide solution, cool, add 200mL of ethyl acetate for extraction, and distill off the ethyl acetate to obtain a yellow oil; the yellow oil is separated by alumina column chromatography, and then 75 % acetonitrile aqueous solution to obtain 11.1 g of light yellow 6-bromomethyl-3-methoxy-2-nitro...
example 3
[0022] Add 15.4g of 6-methyl-3-hydroxy-2-nitropyridine, 16.5g of magnesium iodide and 100g of water into a 250mL four-necked bottle, heat the water bath to 75°C, add dropwise 14.5g of dimethyl sulfate to react 24 hours; add 200mL ethyl acetate for extraction, separate the water layer, evaporate the ethyl acetate, and recrystallize from ethanol to obtain 16.1 g of 6-methyl-3-methoxy-2-nitropyridine. Add 10.1g of 6-methyl-3-methoxy-2-nitropyridine, 10.3g of sodium bromide and 100mL of carbon tetrachloride into a 500mL four-necked bottle, heat to reflux, add 11g of hydrogen peroxide dropwise, and dropwise add Then continue to react for 48 hours; after the reaction, add 100mL of 1M sodium hydroxide solution, cool, add 200mL of ethyl acetate for extraction, evaporate the ethyl acetate to obtain a yellow oil; the yellow oil is separated by alumina column chromatography, and then 75 % acetonitrile aqueous solution to obtain 11.1 g of light yellow 6-bromomethyl-3-methoxy-2-nitropyridi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com