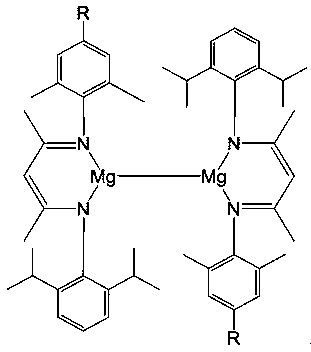
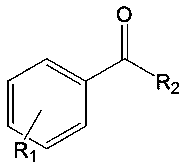
A kind of unsymmetrical β-diimine monovalent magnesium compound and its preparation method and application in hydroboration reaction of aldehydes and ketones
A magnesium compound, asymmetric technology, applied in the preparation of amino compounds, the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve problems such as the hydroboration reaction of borane and carbonyl compounds that have not yet been developed, To achieve the effect of simple and controllable reaction, mild reaction conditions and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1) Asymmetric β-diimine ligand ( DipMes Preparation of Nacnac(H)
[0041]Under the protection of argon, add 120mL of toluene to a 250mL round bottom flask, then add 145.8mmol of 2,6-diisopropylaniline, 145.8mmol of acetylacetone and 1.7mmol of p-toluenesulfonic acid, in the Dean-Stark apparatus Reflux at 160°C for 8h. After the reaction, drain and add 145.8mmol of 2,4,6-trimethylaniline and 145.8mmol of p-toluenesulfonic acid in 120mL of toluene solvent and reflux at 160°C for 24h . Drain, wash with dichloromethane and saturated NaHCO 3 Extracted three times, the organic phase was extracted with anhydrous MgSO 4 After drying and sucking to dryness, the obtained solid is the asymmetric β-diimine ligand with a yield of 64%. NMR data of the product: 1 H NMR (CDCl 3 ,600MHz):δ12.27(s,1H,NH),7.13(s,3H,Ar-H),6.89(s,2H,Ar-H),4.89(s,1H,=CH),3.08(sept , 3 J HH =6.6Hz,2H,CH(CH 3 ) 2 ),2.28(s,3H,CH 3 ),2.16(s,6H,CH 3 ),1.73(s,3H,NCCH 3 ),1.72(s,3H,NCCH 3 ),1.24(d, ...
Embodiment 2
[0045] 1) unsymmetrical β-diimine monovalent magnesium compound [{( DipXyl Nacnac)Mg} 2 ] preparation
[0046] Under anhydrous and oxygen-free conditions, under argon protection, in a single-port reaction tube, 8.29 mmol of methylmagnesium iodide was slowly added dropwise to the asymmetric β-diimine ligand ( DipXyl NacnacH) 8.29mmol ether solution, stirred overnight at room temperature, stood and filtered to obtain magnesium iodide. Then 2.48mmol of magnesium iodide was dissolved in 30mL of toluene, placed in a sodium mirror made of excess sodium 21mmol, reacted for 4 days, filtered, and the filtrate was concentrated to obtain a large number of crystals, which were asymmetric β-dimethoxy Amine monovalent magnesium compound[{( DipXyl Nacnac)Mg} 2 ], the yield was 60%. NMR data of the product: 1 H NMR (C 6 D. 6 ,600MHz):δ7.10–7.00(m,12H,Ar-H),4.84(s,2H,=CH),2.96(sept, 3 J HH =6.6Hz,4H,CH(CH 3 ) 2 ),1.96(s,12H,Ar-CH 3 ),1.57(s,6H,NCCH 3 ),1.48(s,6H,NCCH 3 ),1.10(d,...
Embodiment 3
[0047] Example 3: [{( DipMes Nacnac)Mg} 2 ] Catalyzed synthesis of boronate from benzaldehyde and pinacol borane
[0048] Under anhydrous and oxygen-free conditions, under nitrogen protection, in a glove box, the catalyst [{( DipMes Nacnac)Mg} 2 ]0.0003mmol was added to about 0.5mL of C 6 D. 6 Then add 0.3 mmol of pinacol borane with a pipette gun and mix evenly, and finally add 0.3 mmol of benzaldehyde, react at room temperature for 10 minutes, and measure NMR. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 HNMR (C 6 D. 6 ,600MHz):7.06-7.27(m,5H,Ar-H),4.92(s,2H,Ar-CH 2 ),1.08(s,12H,C(CH 3 ) 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com