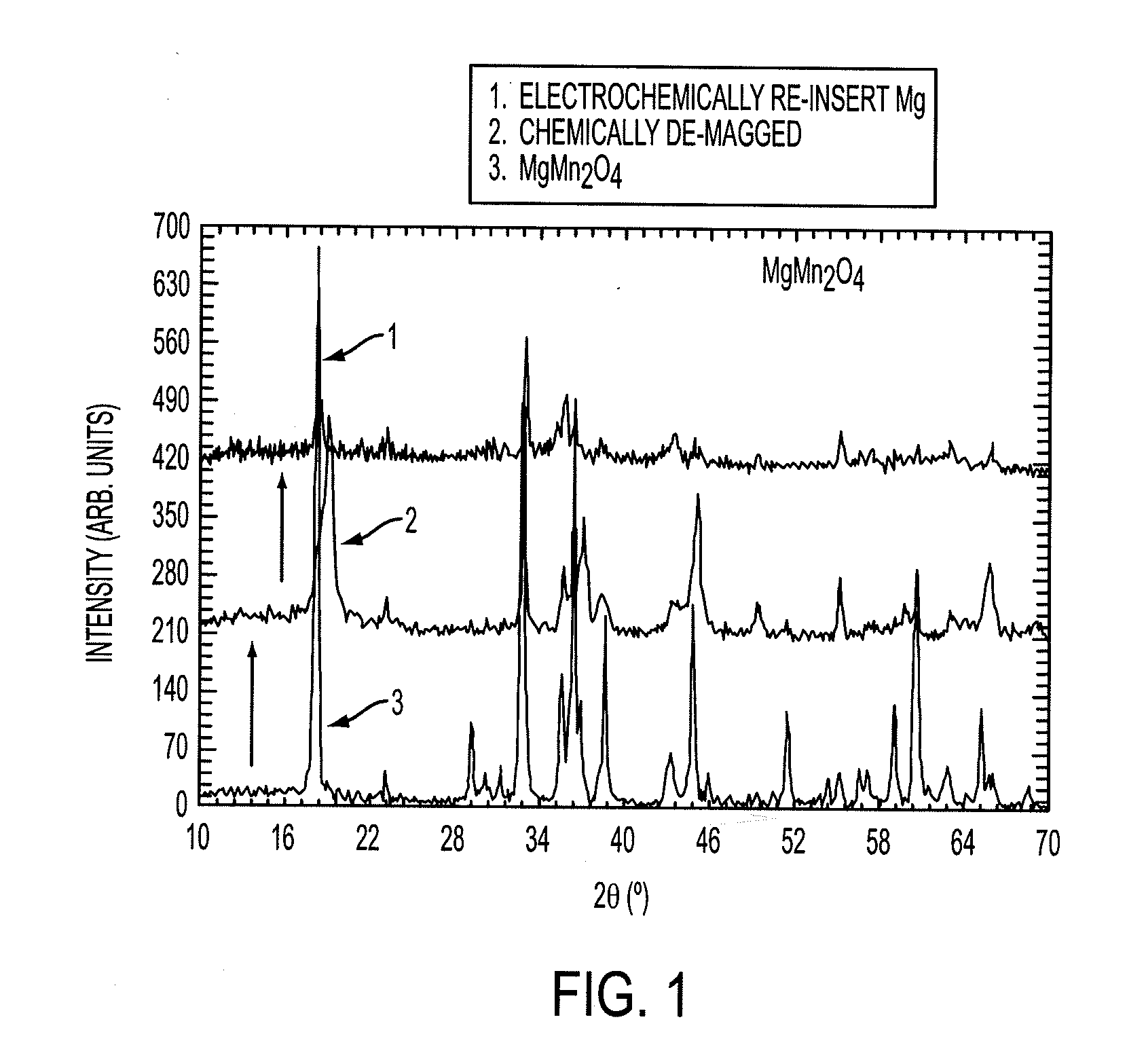
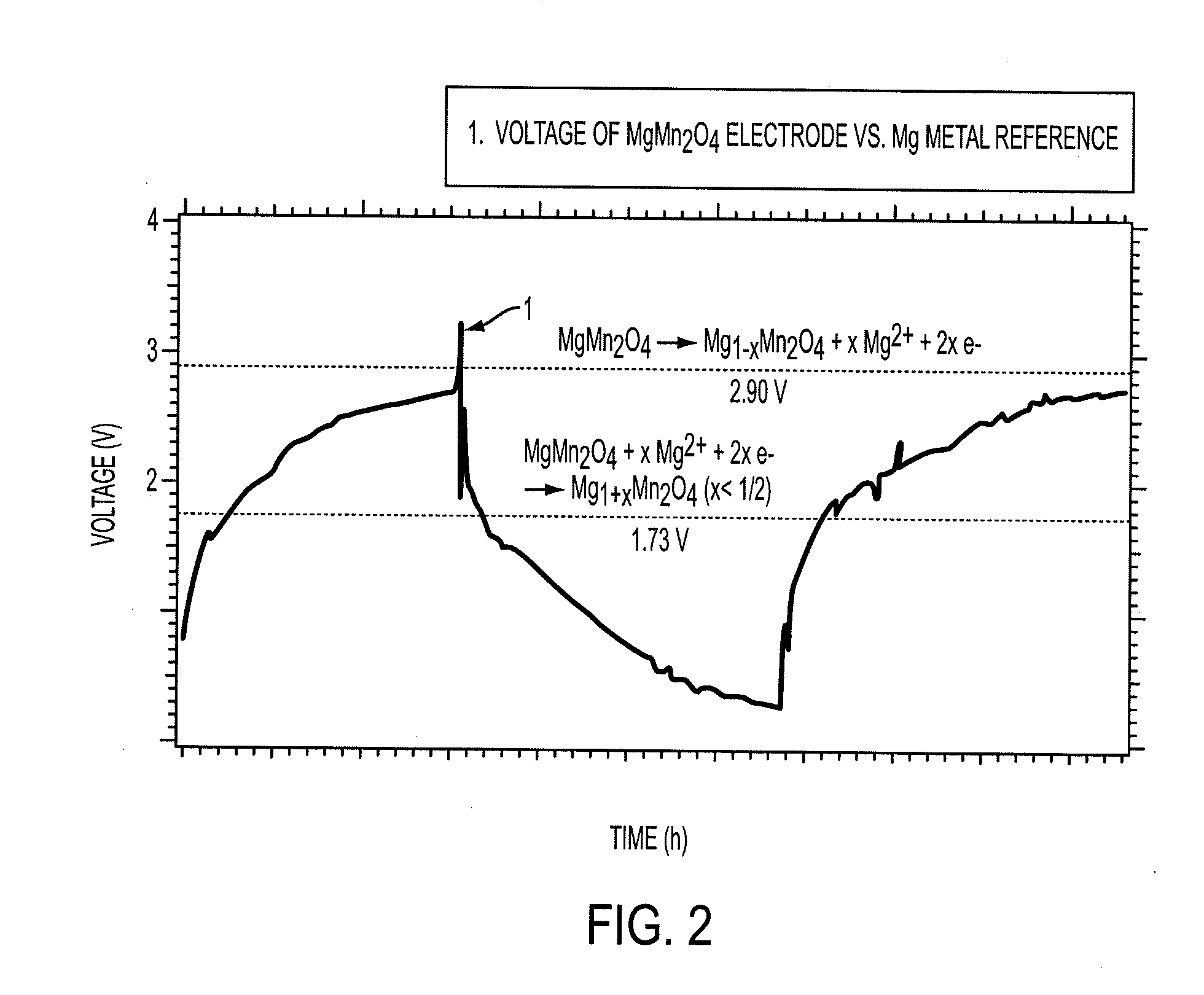
Electrode materials for magnesium batteries
a technology of electrode materials and magnesium batteries, which is applied in the direction of vanadium compounds, cell components, nickel compounds, etc., can solve the problems of failure to commercialize mg batteries, lack of suitable electrode materials, and low barrier to the diffusion of mg, so as to facilitate useful reaction voltage and capacity, and maintain the stability of the host structur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Tables 1 and 2 of Ab Initio Results for Target Materials
[0176]Tables 1 and 2 summarize the composition, structure, and calculated properties for a number of magnesium containing compounds. The compounds exhibit the structure and composition necessary to provide reasonable reaction voltage and Magnesium mobility as computed using a density functional theory framework using the Generalized Gradient Approximation (GGA) with or without a Hubbard correction term (GGA+U), depending on the chemistry involved. The mobility barriers provided in Tables 1 and 2 refer to the minimum activation barrier for an Mg2+ ion to traverse the entire compound unit cell. An activation barrier is defined as the difference in energy between two distinct Mg crystallographic sites and is computed by elastic band calculations. A detailed description of the method and its accuracy can be found in [G. Mills, H. Jonsson, and G. K. Schenter, Surf. Sci. 324, 305, 1995.]. Materials with barriers bXy in the de-magged ...
example 2
Description of Spinel Synthesis
Example 2a
Co-Precipitation Method of MgMn2O4 and MgNiMnO4
[0179]This example demonstrates the synthesis of a high mobility compound with a low diffusive path for magnesium, a new synthetic method was developed which involved a co-precipitation of hydroxide salts, followed by a low temperature calcination process according to the following reactions.
MgSO4(aq)+2MnSO4(aq)+(excess)NaOH(aq)→Mg(OH)2(s)+Mn(OH)2(s)+Na2SO4(aq)(25° C.) [5]
Mg(OH)2(s)+2Mn(OH)2(s)→nano-MgMn2O4(300° C. / 8 hr) [6]
[0180]In a typical synthesis, 2.40 g of MgSO4 and 6.75 g of MnSO4.H2O were dissolved in 100 ml of HPLC water at room temperature in a≦500 ml Erlenmeyer flask. Subsequently, 200 ml of a 1M NaOH solution was slowly added dropwise over 15 minutes with continual stirring at room temperature. Immediately, a brown precipitant formed with the caustic addition and thus was critical to continually stir the solution during the addition to minimize agglomeration. Stirring of the solut...
example 2b
Solid State Synthesis of ZnCr2S4
[0182]Spinels of other chemical classes have also been prepared for materials to be used in Mg electrode active materials. Zinc chromium sulphide was synthesized by solid state reaction methods using stoichiometric amounts of ZnS powder (0.655 g) and Cr2S3 (1.345 g) powder according to the following reaction:
ZnS+Cr2S3→ZnCr2S4900° C. / 12 hr / Argon / tube furnace [9]
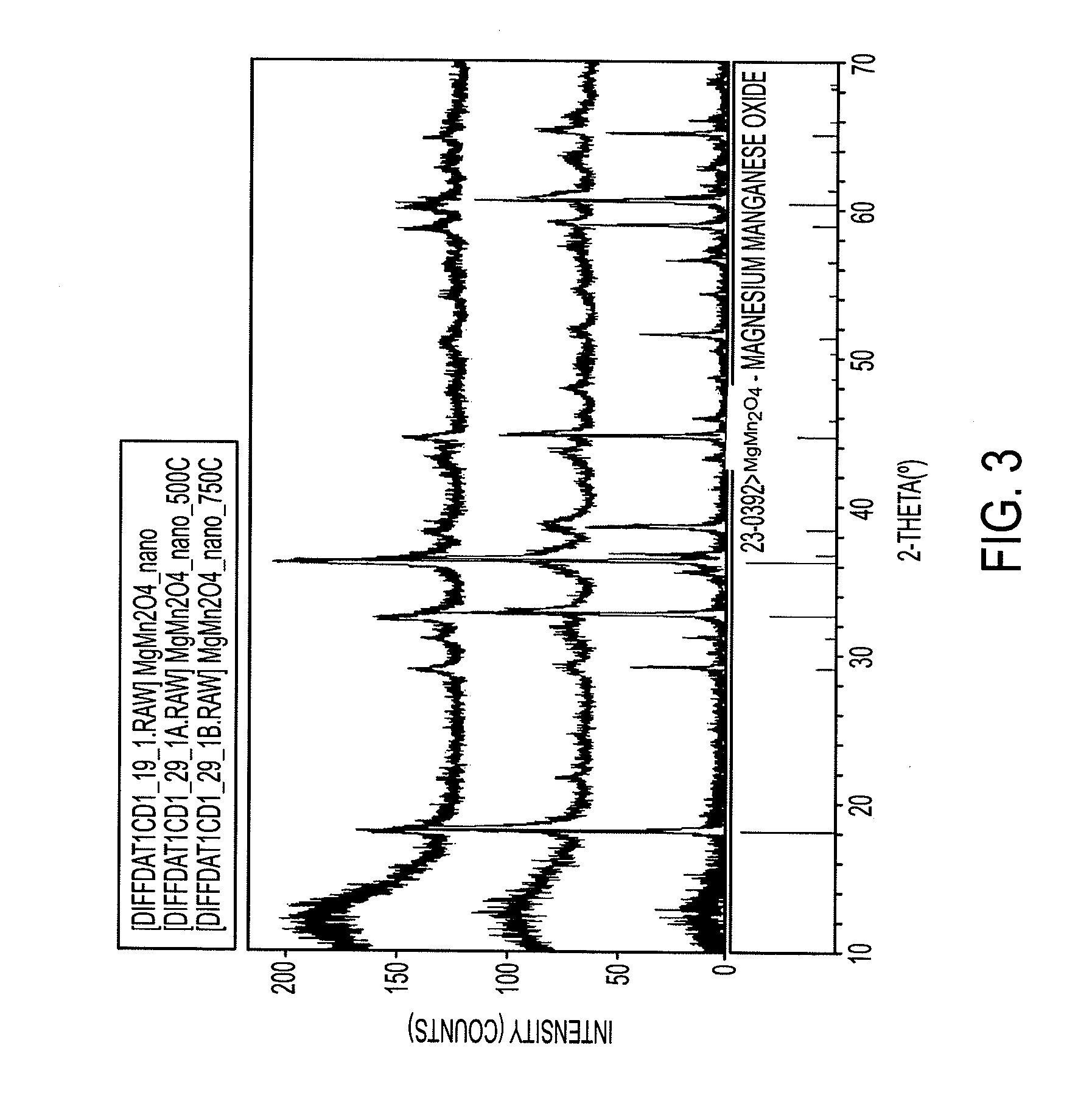
[0183]The mix was pelletized and placed in a tube furnace with flowing argon for 12 hours. XRD analysis showed the product to be relatively phase pure ZnCr2S4 (>95%) as demonstrated in FIG. 5. For those with expertise in the art, synthesis of a variety of high mobility spinel materials can be envisioned using such techniques.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com