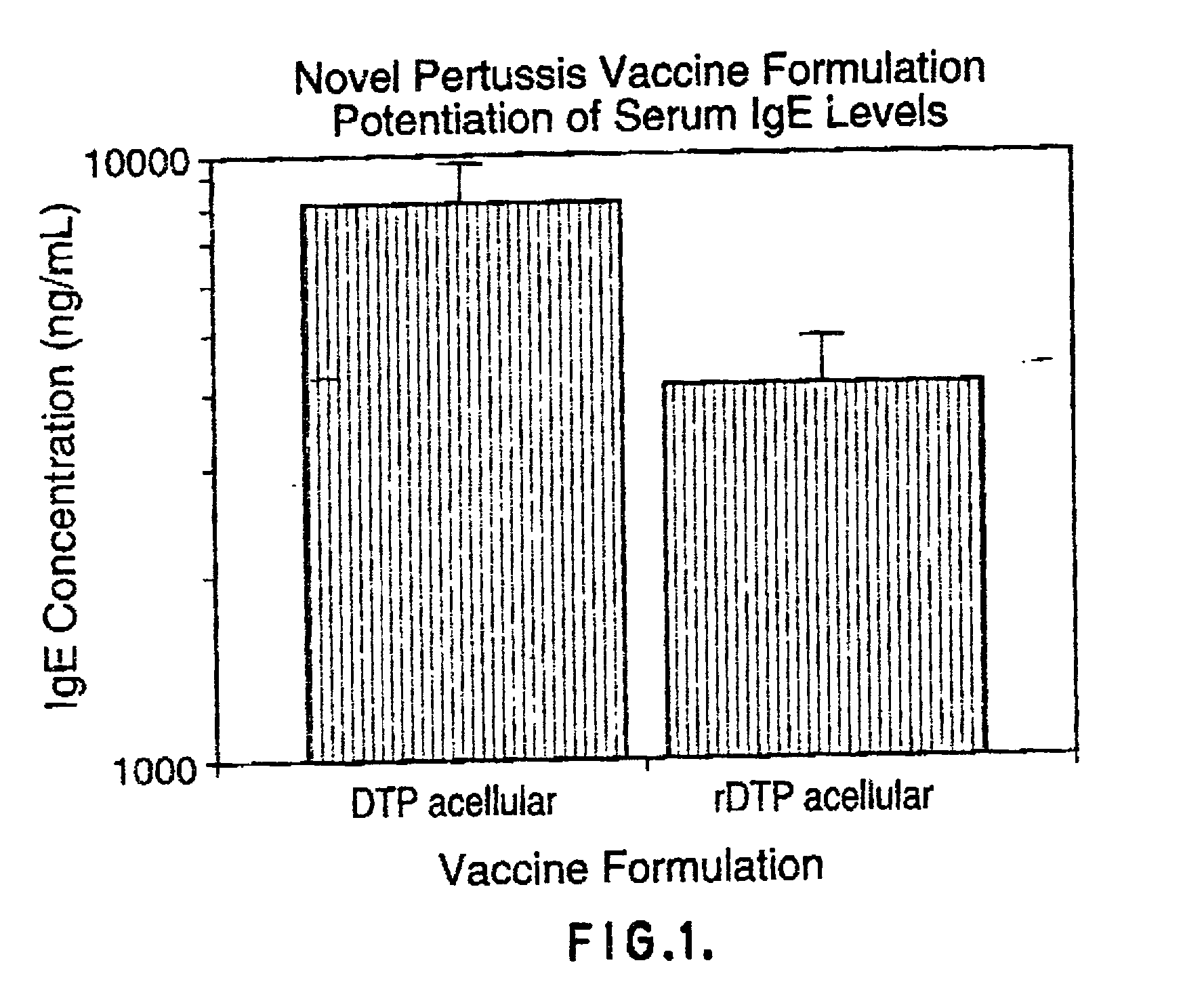
Proteinaceous adjuvants
a technology of proteinaceous adjuvants and adjuvants, which is applied in the direction of antibody medical ingredients, vertebrate antigen ingredients, bacteria antigen ingredients, etc., can solve the problems of ineffective influenza vaccination, inability to induce protective immune responses, and inability to use in humans,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
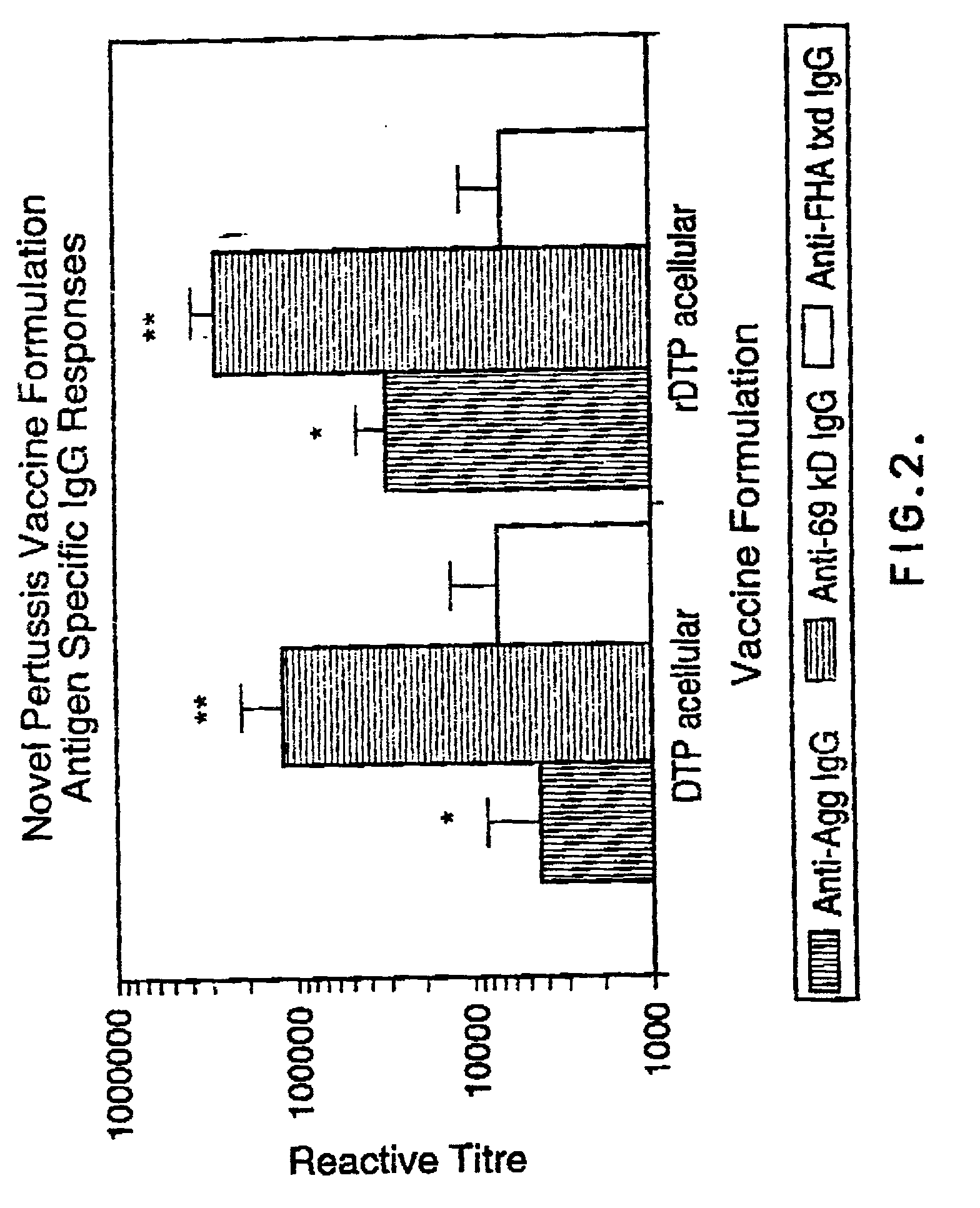
[0071] This Example describes the formulation of vaccines.
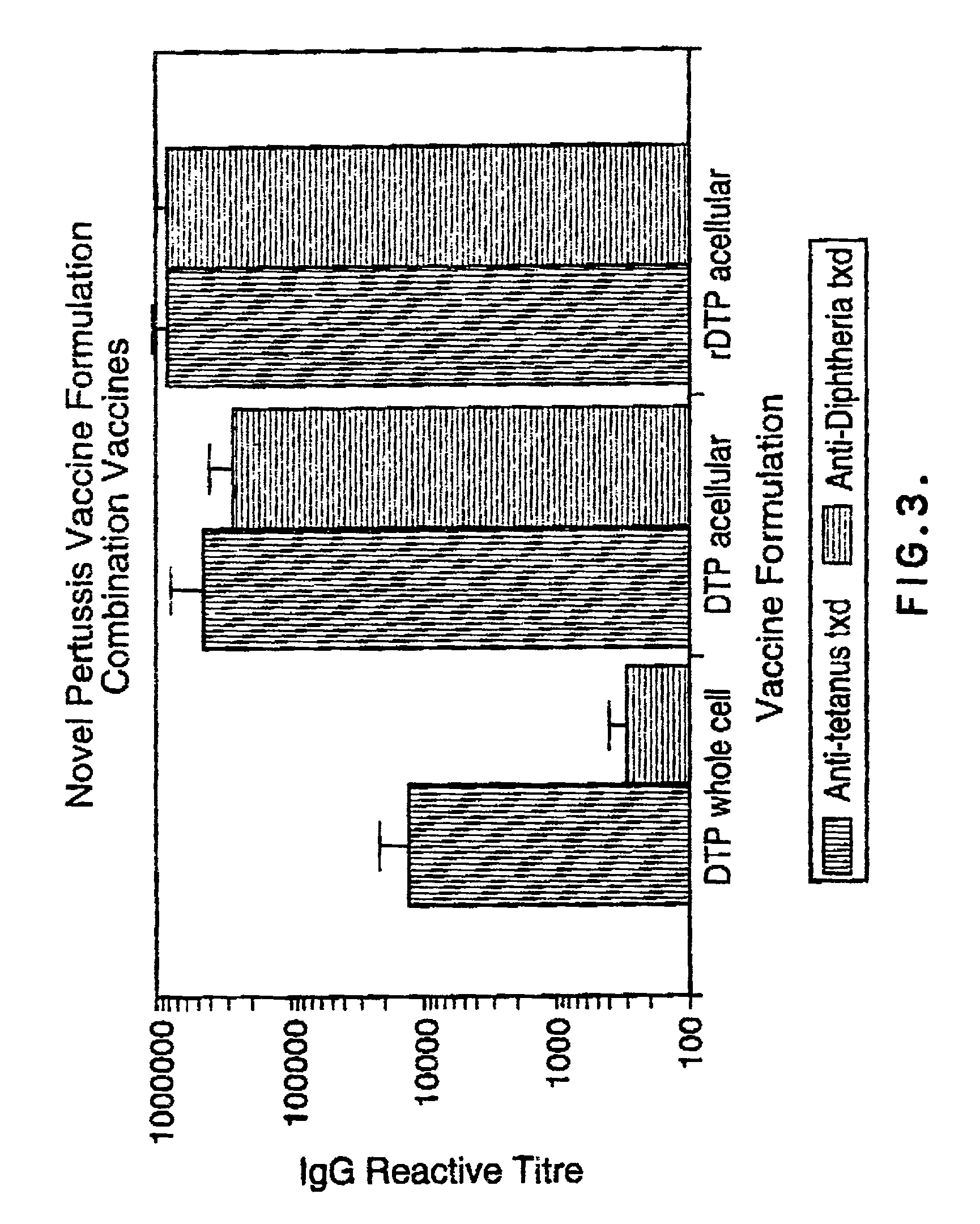
[0072] The DPT whole-cell vaccine and an experimental component acellular vaccine were produced by Connaught Laboratories Ltd. The component acellular pertussis vaccine was alum adsorbed (1.5 mg / dose) and consisted of 10 .mu.g protein nitrogen of glutaraldehyde toxoided pertussis toxin , 5 .mu.g protein nitrogen each of FHA and agglutinogens 2 and 3 and 3 .mu.g of pertactin along with 5 Lf of tetanus toxoid and 25 Lf of diphtheria toxoid per dose. The recombinant component vaccine also contained 5 .mu.g protein nitrogen each of FHA and agglutinogens 2 and 3 and 3 .mu.g protein nitrogen of pertactin in addition to 5 Lf of tetanus toxoid and 25 Lf of diphtheria toxoid per dose. However, it varied from the other acellular vaccine in that it contained 20 .mu.g protein nitrogen of the recombinant PT mutant holotoxin, K9G129, and was not alum adsorbed. The K9G129 pertussis toxin molecule as well as the purified FHA, agg 2+3 and per...
example 2
[0073] This Example describes immunization of animals.
[0074] Female BALB / c mice weighing 15 to 18 grams were obtained from Charles River Canada (St. Constant, Quebec). The mice were housed in microisolators and used in accordance with the guidelines set by the Canadian Council on Animal Care (CCAC). The animals were specific pathogen free and the housing rooms were monitored for Murine Hepatitis virus outbreaks through the use of sentinel mice. Water was provided ad libitum and the diet was ovalbumin-free. The mice were immunized on Day 0 with the vaccine formulations in groups of six. A booster dose of vaccine was administered an Day 21. On Day 28 the animals were bled via jugular vein laceration and spleneostomized. The serum samples were stored at -20.degree. C. until assayed.
example 3
[0075] This Example describes antigen specific imunuoassays.
[0076] The vaccine antigen specific IgG responses were determined by indirect EIA. The antigens of interest were pertussis toxin, pertactin, filamentous hemagglutinin, agglutinogens, as well as diphtheria and tetanus toxoids. High binding capacity microplates (Nuno) were coated with 4 .mu.g / mL of each of the above antigens in a volume of 50 uls / well of 50 mM carbonate buffer pH 9.6. After an overnight incubation, the plates were washed and successively blocked with a 0.1% solution of bovine serum albumin (sigma) for one hour at room temperature. The excess block was removed and the microplates were washed. The murine serum samples were then serially diluted in PBS-Tween 20 (0.05%) and plated out at a volume of 100 uls. The samples were incubated overnight at 4.degree. C. The antigen specific fraction of IgG antibodies was detected by a peroxidase conjugated sheep anti-mouse IgG conjugate (Jackson Laboratories). The plates w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com