A pertussis vaccine microneedle array and preparation method thereof
A microneedle array, pertussis technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve the problem of inaccurate measurement, achieve excellent immune effect, avoid spillage, and operate safely. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing a pertussis vaccine microneedle array, comprising the steps of:
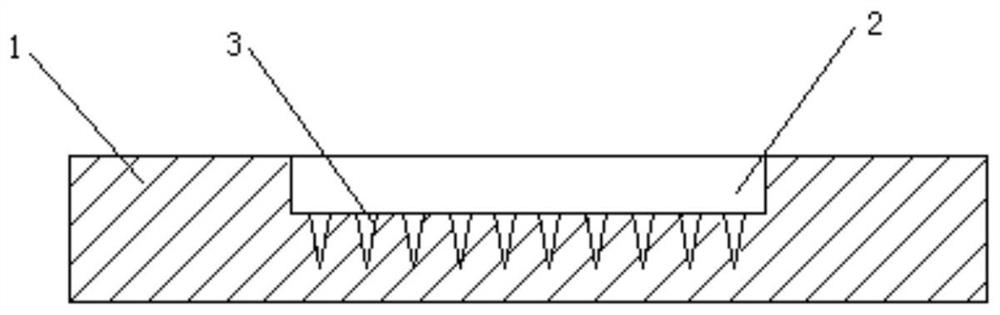
[0027] 1) Using a microneedle array mold, see figure 1 , the microneedle array mold includes a substrate 1, a rectangular groove 2 is arranged on the upper surface of the substrate 1, and a 10×10 array of tapered grooves 3 with downward pointed points is arranged on the bottom wall of the rectangular groove 2, and each cone The volume of the shaped groove 3 is 0.02 μL, and the total volume of the tapered groove is 2ul;
[0028] 2) Mix 1 mg / mL inactivated pertussis toxin aqueous solution and 10 mg / mL CpG adjuvant aqueous solution at a volume ratio of 1:1 to obtain solution 1, absorb 100 microliters and add it to rectangular groove 2 , stand still under vacuum conditions for 15 minutes, make the solution fill the conical groove 3, absorb excess solution other than the conical groove 3, and dry for 60 minutes;
[0029] 3) Smear a polyvinyl alcohol aqueous solution with a mass concent...
Embodiment 2
[0034] A method for preparing a pertussis vaccine microneedle array, comprising the steps of:
[0035] 1) with embodiment 1 step 1);
[0036] 2) Mix 0.1 mg / mL inactivated pertussis toxin aqueous solution and 10 mg / mL CpG adjuvant aqueous solution at a volume ratio of 1:1 to obtain solution 1, absorb 100 microliters and add it to the rectangular groove In 2, stand still under vacuum condition for 15 minutes, make the solution 1 fill the conical groove 3, absorb excess solution 1 other than the conical groove 3, and dry for 60 minutes;
[0037] 3) Same as embodiment 1 step (3).
[0038] Each pertussis vaccine microneedle array was loaded with pertussis toxin 0.1 μg and CpG adjuvant 10 μg.
Embodiment 3
[0040] A method for preparing a pertussis vaccine microneedle array, comprising the steps of:
[0041] 1) with embodiment 1 step 1);
[0042] 2) Mix 0.5 mg / mL inactivated pertussis toxin aqueous solution and 10 mg / mL CpG adjuvant aqueous solution at a volume ratio of 1:1 to obtain solution 1, absorb 100 microliters and add it to the rectangular groove In 2, stand still under vacuum condition for 15 minutes, make the solution 1 fill the conical groove 3, absorb excess solution 1 other than the conical groove 3, and dry for 60 minutes;
[0043] 3) Same as embodiment 1 step (3).
[0044] Each pertussis vaccine microneedle array was loaded with 0.5 μg of pertussis toxin and 10 μg of CpG adjuvant.
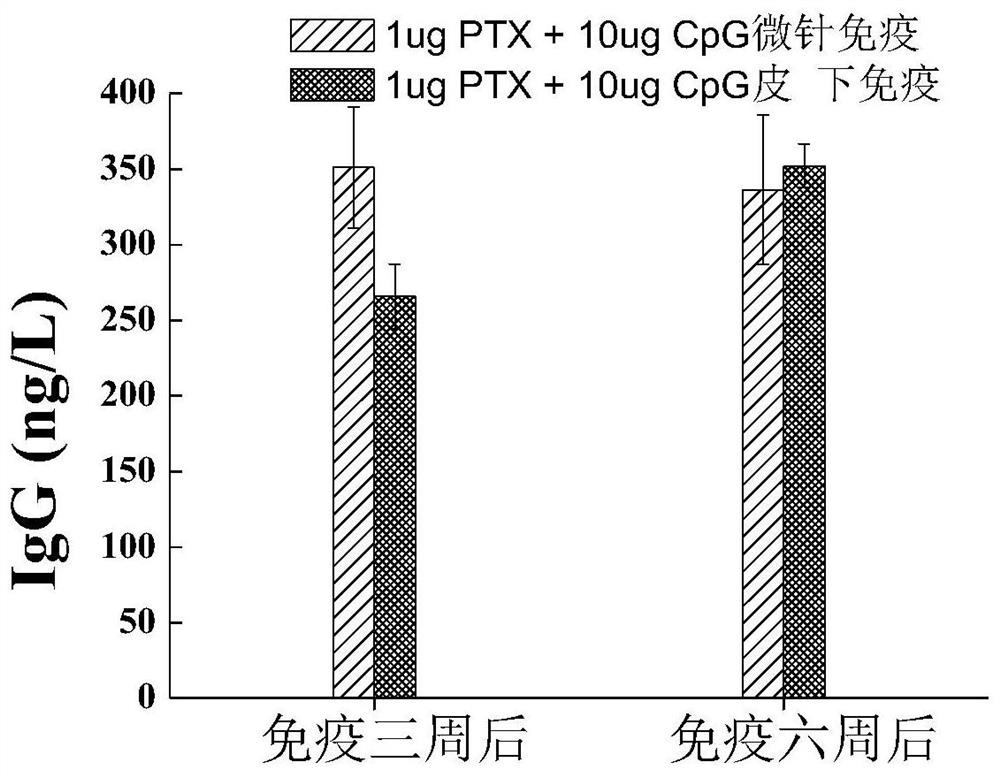
[0045] Experiment 2:
[0046] 24 female Balb / c mice, weighing 18-20g, were randomly divided into 4 groups, 6 in Example 1 group, and intradermally immunized with the pertussis vaccine microneedle array in Example 1; 6 in Example 2 group, respectively Intradermal immunization with th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com