Bordetella pertussis PT (pertussis toxin) antigen monoclonal antibody and application thereof
A monoclonal antibody and pertussis technology, applied in the field of immunology and vaccinology, can solve the problems of poor specificity and low detection efficiency of PT antigen content detection methods, and achieve the effects of reducing experimental costs, high titers, and high immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 immunogen preparation and animal immunization
[0041] (1) Bacteria pertussis 58003 strain (sourced from China Medical Bacteria Collection and Management Center) was fermented with ginger-packed medium.
[0042] (2) After fermentation, add formaldehyde solution to the final concentration of 0.5% in the fermented cells to sterilize.
[0043] (3) Centrifuge at 4000 rpm or more for more than 10 minutes, discard the supernatant, collect the bacteria, and blow the bacteria with PBS / 0.5% formaldehyde.
[0044] (4) Grinding the bacteria, resuspending and diluting with PBS / 0.5% formaldehyde after grinding.
[0045] (5) Mix the above-mentioned resuspended bacteria with Freund's complete adjuvant in equal volumes, and immunize BALB / c mice with the emulsion formed on day 0, day 14, and day 28, 0.2ml / mouse.
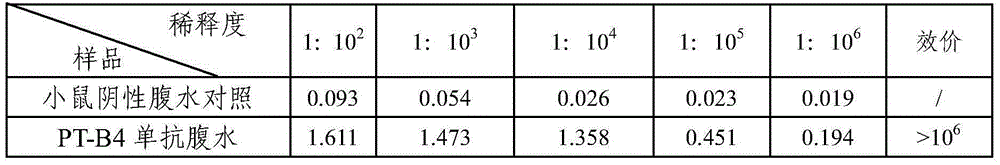
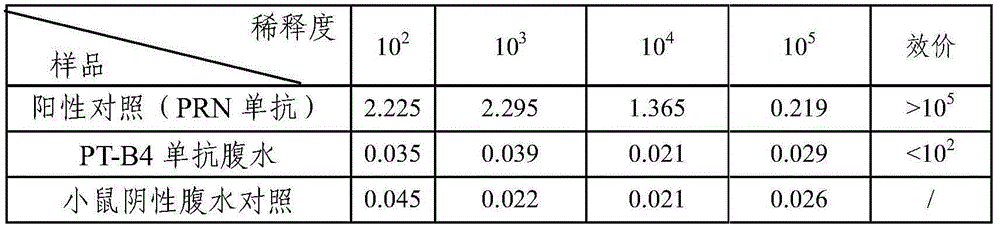
[0046] (6) One week after the last immunization, blood test antibody titer, if higher than 10 3 , 1 week after blood collection, 0.2 μg of emulsion was used for ...
Embodiment 2
[0047] Example 2 cell fusion
[0048] (1) Two weeks before cell fusion, the SP2 / 0 cell line was revived and cultured, expanded 3 days before fusion, and RPMI1640 cell culture medium (Gibco) was removed 1 day before fusion, and culture medium was added again.
[0049] (2) The mice were sacrificed after 3 days of intraperitoneal shock, and the mouse splenocyte suspension was prepared according to conventional methods.
[0050] (3) Add appropriate amount of incomplete IMDM medium (Gibco) to splenocytes and myeloma cell SP2 / 0 according to the counting results, shake and mix the SP2 / 0 cells, and pipette the splenocytes evenly.
[0051] (4) Mix splenocytes and SP2 / 0 cells in a 1:1 ratio in a 50ml centrifuge tube, and mix well.
[0052] (5) Add incomplete IMDM culture medium to 50ml, centrifuge for 5 minutes, pour off the supernatant as much as possible, and use a pipette to suck up the liquid from the nozzle.
[0053] (6) Lightly tap the bottom of the fusion tube to loosen the pre...
Embodiment 3
[0063] Cloning of embodiment 3 hybridoma cells (limiting dilution method)
[0064] (1) Hybridoma cells were counted, and the hybridoma cells were diluted with HT medium containing 20% serum.
[0065] (2) Diluted hybridoma cells were added to a 96-well plate for culture, 100 μl per well.
[0066] (3) 37°C, 5% CO 2 Wet culture for 8 days, when clones visible to the naked eye appear, detect antibody activity in time. Observe under an inverted microscope, mark the wells where only a single clone grows, and take the supernatant for antibody detection.
[0067] (4) Move the cells in the positive wells to a 24-well plate for expanded culture, and then transfer to a 25 or 175 cell bottle for expansion.
[0068] (5) Freeze the cell lines as soon as possible after amplification, number them, and store them in liquid nitrogen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com