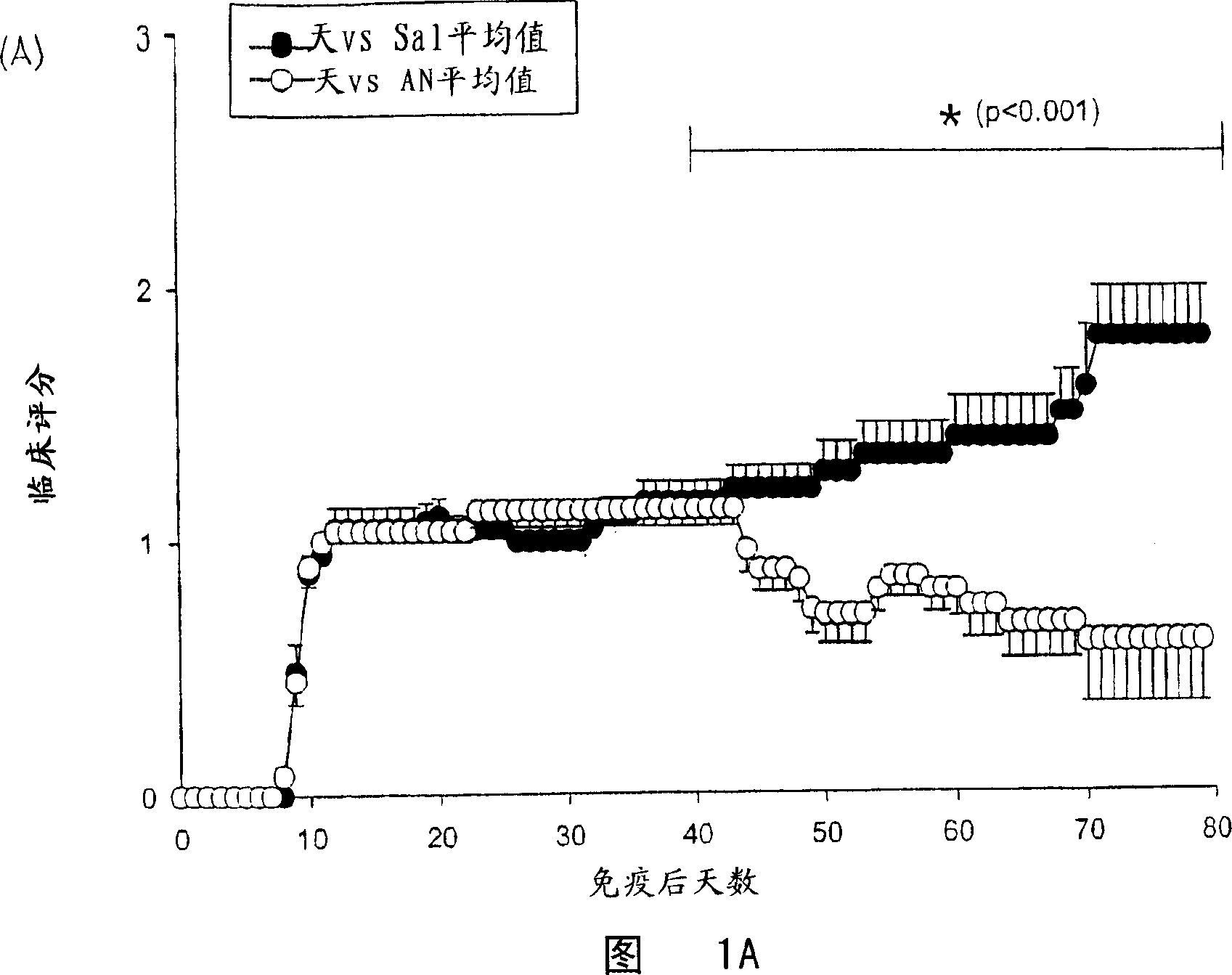
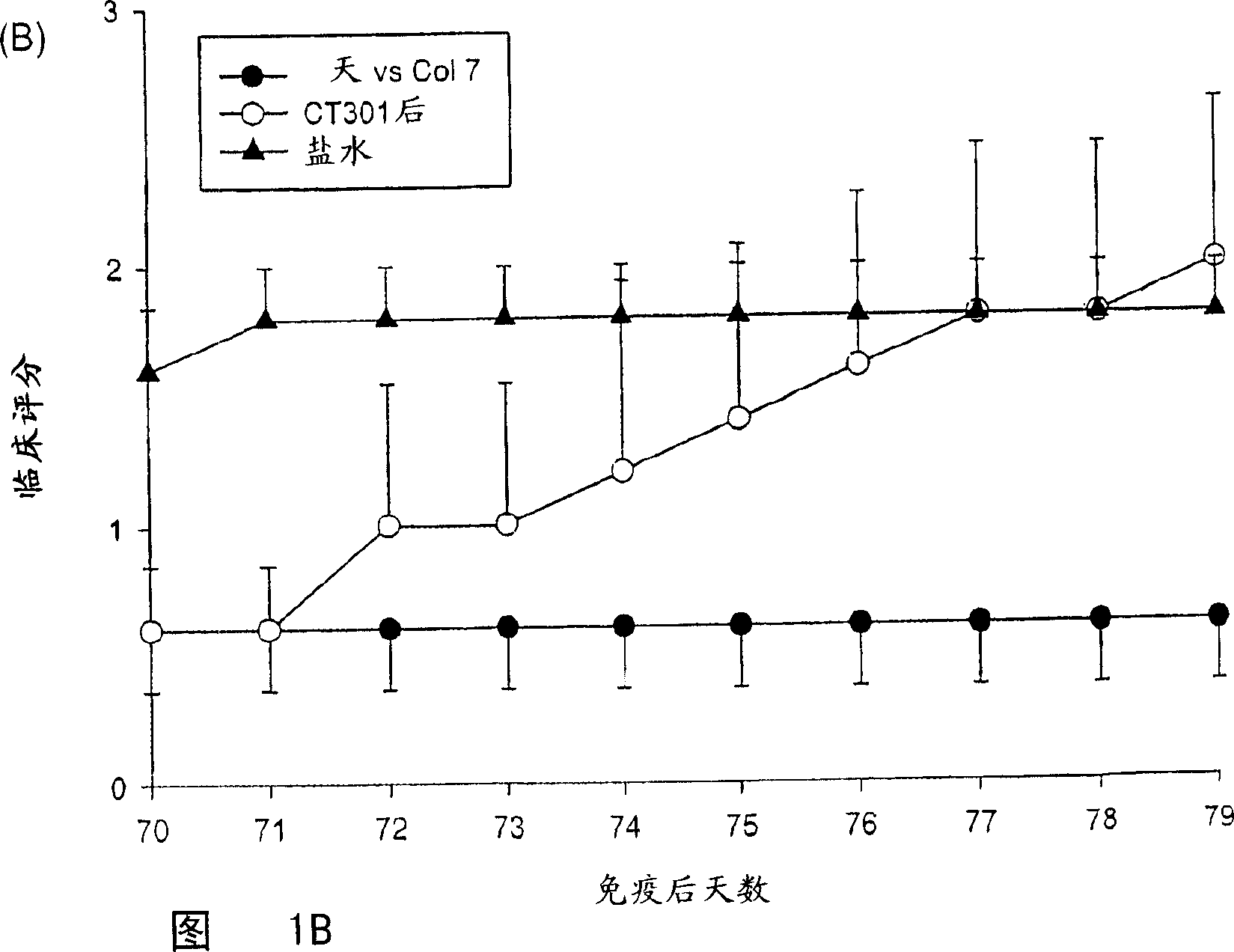
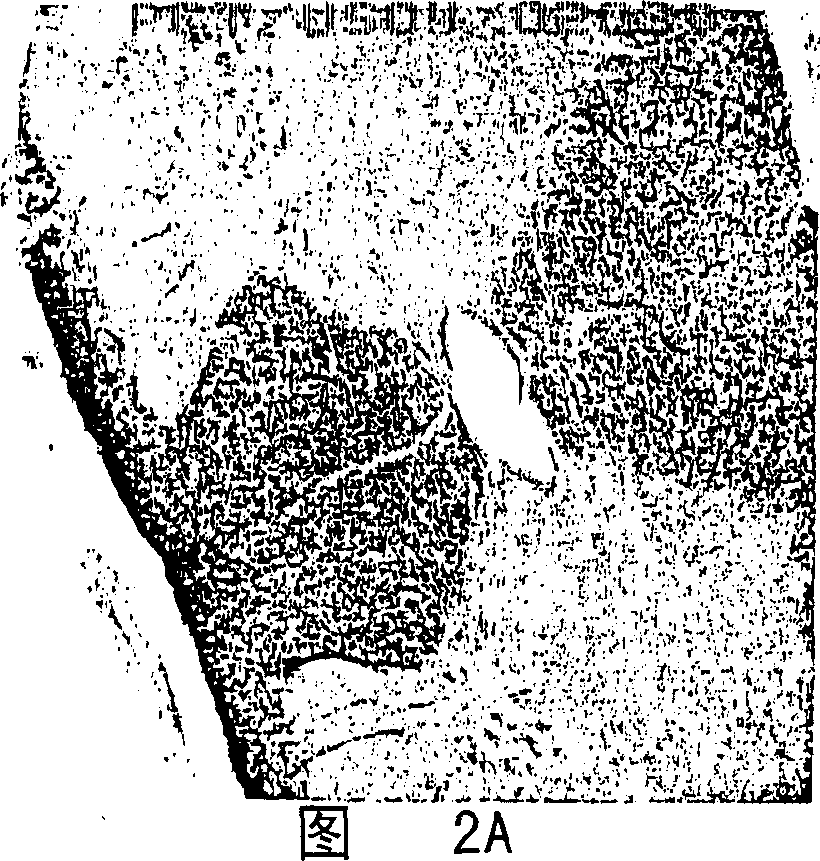
Composition for and treatment of demyelinating diseases and paralysis by administration of remyelinating agents
A kind of technology of remyelination and demyelination, used in demyelinating diseases and disorders and/or the composition and compound field of reducing paralysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1301] Compound preparation
[1302] The compounds of the present invention can be easily prepared from available starting materials using the following general-purpose process. It should be understood that although typical or preferred process conditions (ie, reaction temperature, time, molar ratio of reactants, solvent, pressure, etc.) are given, other conditions may also be used unless otherwise specified. The optimal reaction conditions can vary according to the specific reactants or solvents used, but those skilled in the art can determine these conditions by providing routine optimization procedures.
[1303] In addition, those skilled in the art should understand that conventional protecting groups may be required to prevent undesirable reactions of certain functional groups. Protecting groups suitable for different functional groups and conditions suitable for the protection and deprotection of particular functional groups are well known in the art. For example, various pr...
Embodiment 1
[1395] Prepare hard gelatin capsules containing the following components:
[1396] quality
[1397] Ingredients (mg / capsule)
[1398] Active component 30.0
[1399] Starch 305.0
[1401] The above components were mixed and filled into hard gelatin capsules at 340 mg of bright.
Embodiment 2
[1403] The following components are used to prepare tablets:
[1404] quality
[1405] Ingredients (mg / capsule)
[1406] Active ingredient 25.0
[1407] Cellulose, microcrystalline 200.0
[1408] Colloidal silica 10.0
[1409] Stearic acid 5.0
[1410] The components are mixed and compressed into tablets, each weighing 240 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com