Streptococcus protective antigen SAP and preparation method thereof
A protective antigen, streptococcus technology, applied in biochemical equipment and methods, chemical equipment and methods, viruses/bacteriophages, etc., can solve problems such as difficult to effectively control SEZ infection, and achieve high protective efficacy and strong specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of the above-mentioned streptococcal protective antigen SAP is as follows:
[0030] 1) PCR amplification: use the SEZ MGCS10565 genome (NCBI Accession: CP001129.1) as a template, and use primers for PCR amplification. The primers are:
[0031] Forward primer (SEQ ID NO.3): 5′-CATGCG GGATCC CAAGTA -3′, the underlined part is BamHI Restriction sites;
[0032] Reverse primer (SEQ ID NO.4): 5′-AATCTTCTGGTCGACACTT-3′, the underlined part is Sal I Restriction sites.
[0033] 2) Ligation with the vector: the PCR product was digested with a restriction endonuclease, and the recovered PCR digest product was ligated with the pET-32a vector to obtain a recombinant vector.
[0034] 3) Transformation and induction: After transforming E. coli with the recombinant vector, incubate until the exponential growth phase, and continue to incubate for 3 hours after adding IPTG.
[0035] Preferably, the Escherichia coli is BL21 competent cells; the added IPTG i...
Embodiment 1
[0042] Example 1 Determination of antibody titer
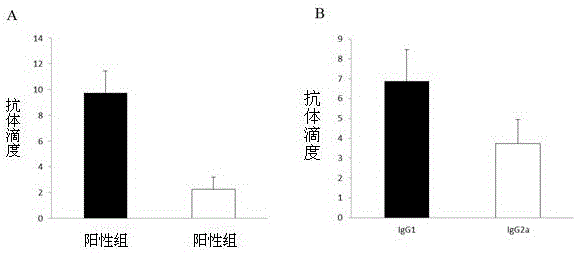
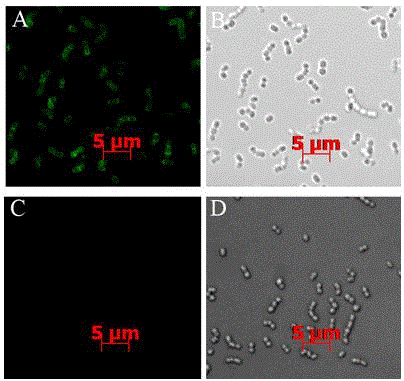
[0043] Serum IgG titer was measured by ELISA, and the purified recombinant protein SAP was coated on the enzyme-linked plate, and the blood was collected from the mice 10 days after the second immunization to separate the serum. After serial dilution, 100 μL of each dilution was added to the enzyme-linked plate. After reacting at 37°C, add rabbit anti-mouse IgG, read the OD value with a microplate reader, and take the maximum dilution factor of the serum whose OD value is greater than the average OD value of the control serum + 3 times the variance SD (standard deviation) as the serum antibody. In order to infer the immune type, IgG1 and IgG2a were further used as controls to measure Th2 and Th1 immune responses, respectively.
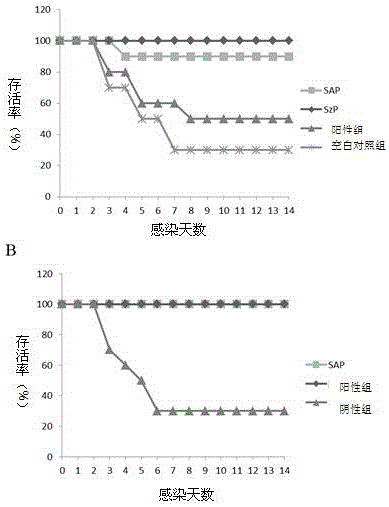
[0044] Experimental results: the IgG titer level of the immune group was significantly higher than the antibody level of the negative control group (p figure 1 A).
[0045]The results showed that SAP...
Embodiment 2
[0046] Example 2 Mice immunization and challenge test
[0047] 1. Forty 4-week-old BALB / c female mice were randomly divided into 4 groups, 10 in each group;
[0048] 2. Experimental group: After 50 μg of purified recombinant SAP protein was emulsified with Marcol 52 adjuvant, the mice in the first group were immunized by intraperitoneal injection, and the mice were immunized for the second time in the same way 14 days later;
[0049] 3. Positive control group: After 50 μg of purified recombinant SzP protein was emulsified with Marcol 52 adjuvant, the mice in group 1 were immunized by intraperitoneal injection, and immunized mice were injected for the second time in the same way 14 days later;
[0050] 4. Negative control group: inject PBS emulsified with Marcol 52 adjuvant to mice in group 3;
[0051] 5. Blank control group: inject PBS to the mice in group 4;
[0052] 6. Ten days after the second immunization injection of all mice, the blood was collected from the tail vein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com