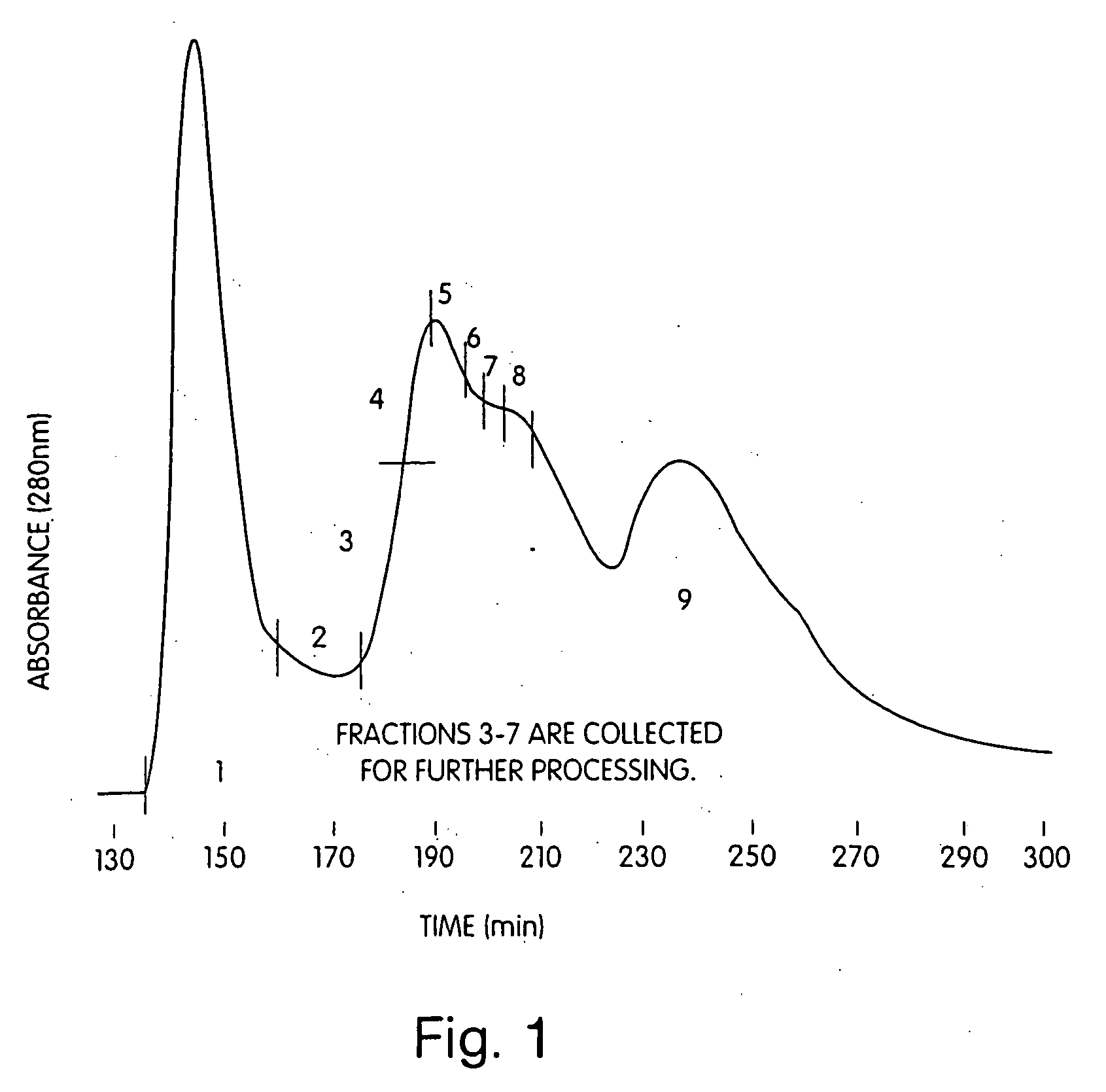
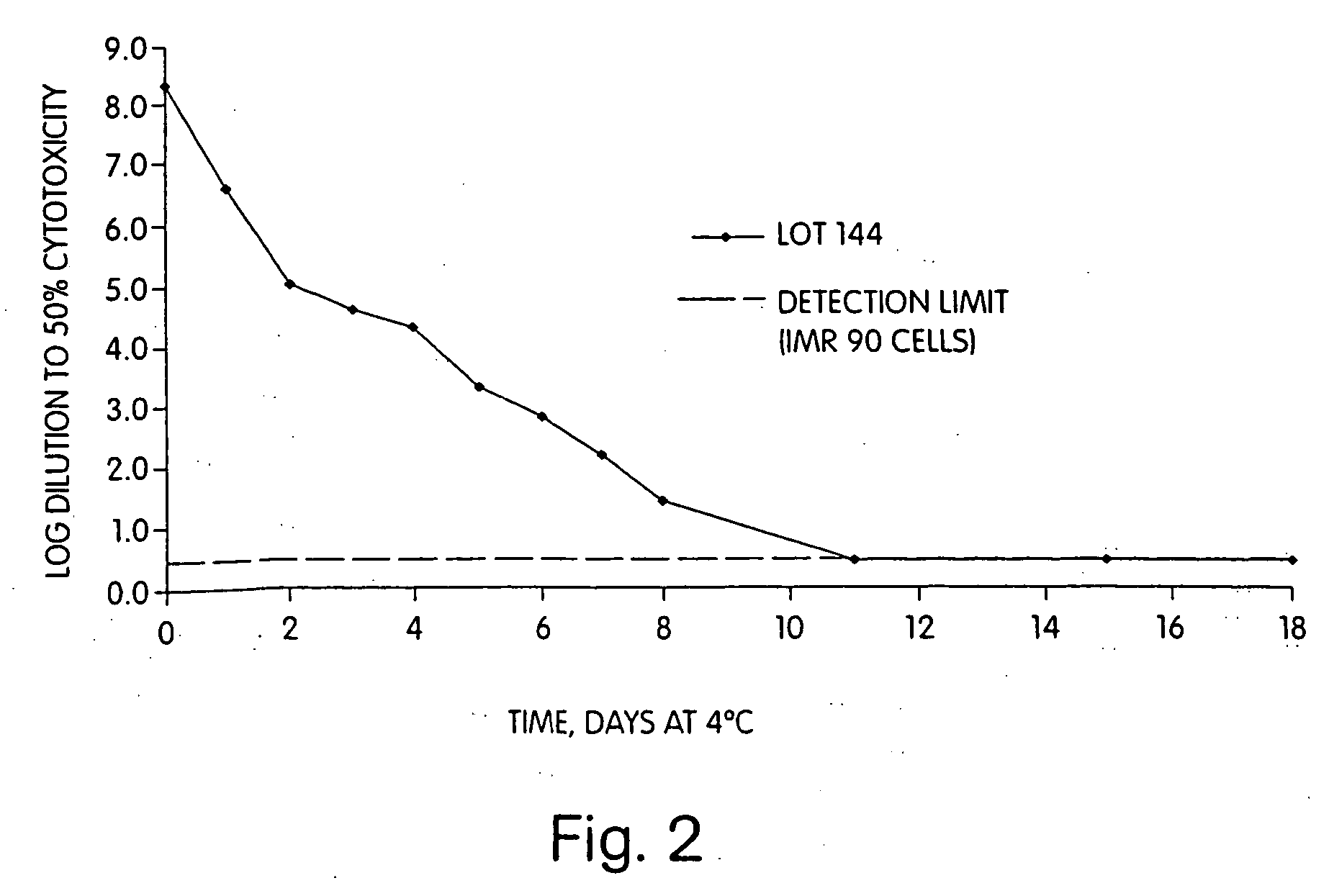
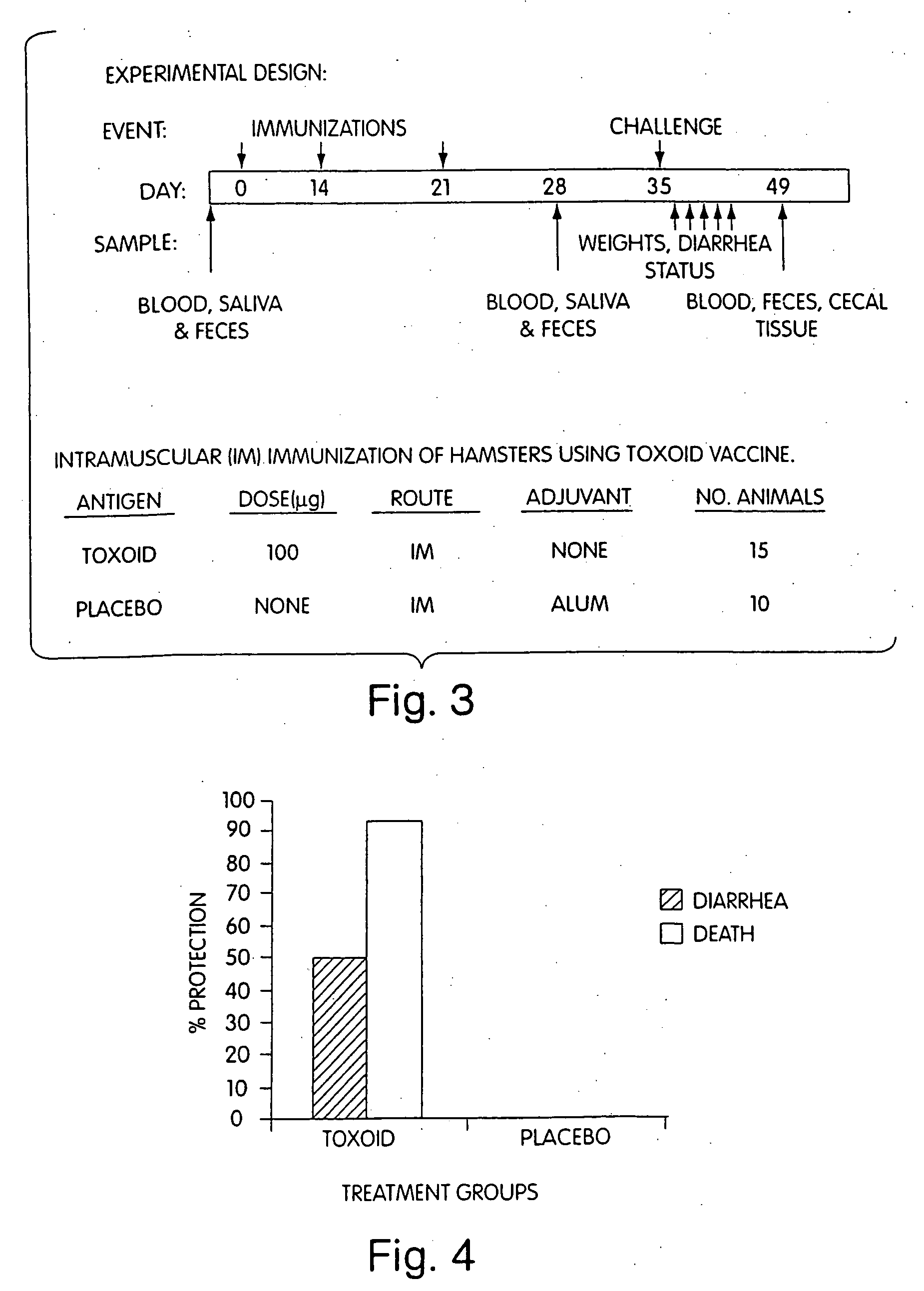
Immunization against clostridium difficile disease
a technology of clostridium difficile and immune response, which is applied in the field of immunomodulation against clostridium difficile disease, can solve the problems of lethal both toxins to animals, and achieve the effect of preventing relapse and facilitating immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The invention provides methods and compositions for preventing and treating C. difficile disease in mammals, such as humans. The methods include passive and active immunization approaches, which involve percutaneous (e.g., intramuscular, intravenous, or intraperitoneal) administration of antibodies (e.g., toxin-neutralizing polyclonal immune globulin) to C. difficile toxoids, C. difficile toxoids themselves, or combinations thereof. For example, the invention includes methods of preventing and / or treating recurrent C. difficile associated diarrhea (CDAD) by percutaneous administration (e.g., intramuscular) of a vaccine including toxoid A and / or toxoid B. The invention also includes C. difficile toxoids, vaccine compositions containing C. difficile toxoids, methods of producing C. difficile toxin-neutralizing polyclonal immune globulin, substantially purified C. difficile toxin-neutralizing polyclonal immune globulin, and methods of identifying donors of C. difficile toxin-neu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com