SARS-CoV-2 neutralization antibody detection kit
A detection kit, sars-cov-2 technology, applied in the direction of biological testing, measuring devices, immunoassays, etc., can solve the problems of low sensitivity, inability to accurately detect antibodies, and inability to realize large-scale screening, so as to improve sensitivity and specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of RBD recombinant protein
[0040] The RBD domain gene of SARS-CoV-2 (306-527 amino acid sequence of S protein (GeneID: 43740568)) was transformed into competent Escherichia coli DH5α, ice-bathed for 30 minutes, and cultured in LB medium at 37°C for 60 minutes Recover, and finally take the bacterial solution and cultivate it on the LB agar plate containing Amp antibiotics to select positive colonies for identification;
[0041] Select the correctly recombined plasmid to transform Escherichia coli DH10 Bac competent cells, recover for 2-4 hours, and culture in LB medium containing Kan+Gent+Tet to select positive colonies for identification;
[0042] Transform the correctly recombined bacmid into insect cell sf9, centrifuge to collect the supernatant after 3-5 days, and use it as the first-generation virus; re-infect the insect cell sf9 with the first-generation virus, collect the supernatant after 2-4 days, and use it as the second-generation virus The s...
Embodiment 2
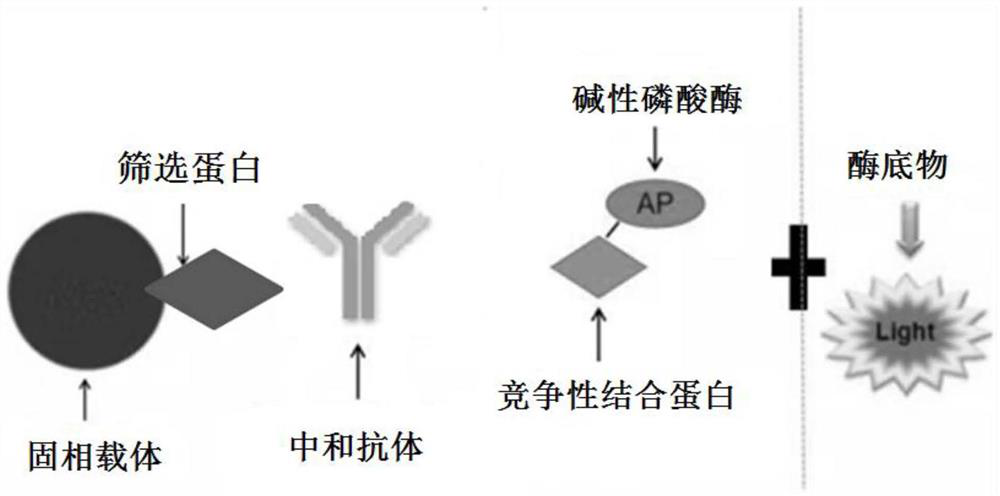
[0051] This embodiment provides a SARS-CoV-2 neutralization detection kit 1, including M reagent, R2 reagent, R1 reagent and calibrator.
[0052] M reagent: the recombinant RBD protein prepared in Example 1 was coated on the surface of the tosyl magnetic beads, and the tosyl magnetic beads coated with the recombinant RBD protein were diluted to a working concentration of 0.5ug / mL with PBS buffer (in the form of recombinant RBD protein concentration meter);
[0053] R2 reagent: label alkaline phosphatase (AP) on the surface of ACE2 antigen, use Sulfo-SMCC and 2-IT as the joint cross-linking agent, dilute the ACE2 antigen labeled AP enzyme to 1ug / mL working concentration with MES buffer;
[0054] R1 reagent: add a stabilizer to the PBS buffer solution, the stabilizer is 1% of the total mass of the R1 reagent glycine and 0.5% of the total mass of the R1 reagent Tween 20.
[0055] Calibrator: Use the recombinant RBD protein prepared in Example 1 as an immunogen to screen out high...
Embodiment 3
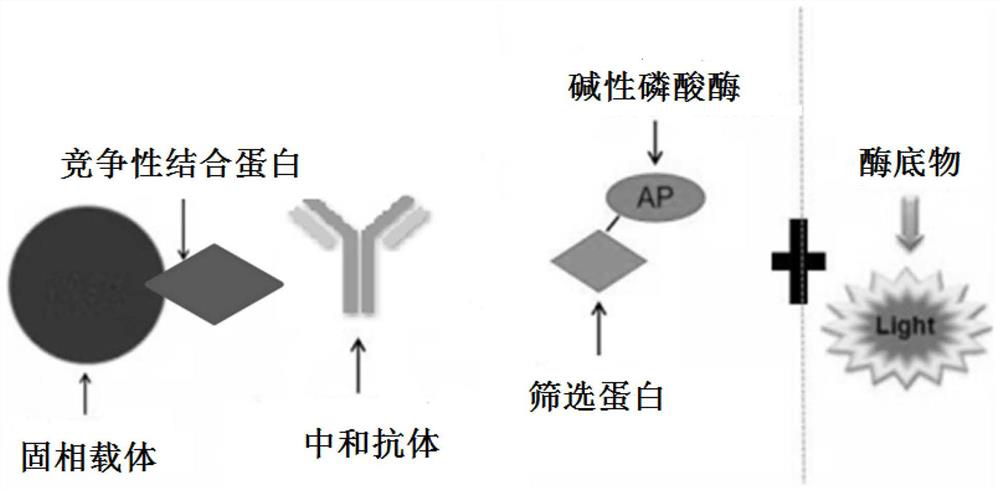
[0057] This embodiment provides a SARS-CoV-2 neutralization detection kit 2, including M reagent, R2 reagent, R1 reagent and calibrator.
[0058] M reagent: ACE2 antigen is coated on the surface of tosyl magnetic beads, and the tosyl magnetic beads coated with ACE2 antigen are diluted to a working concentration of 0.5ug / mL (based on the concentration of ACE2 antigen) with PBS buffer;
[0059] R2 reagent: the recombinant RBD protein surface labeled alkaline phosphatase (AP) prepared in Example 1, using Sulfo-SMCC and 2-IT as a joint cross-linking agent, using MES buffer to dilute the recombinant RBD protein labeled AP enzyme into 1ug / mL working concentration;
[0060] R1 reagent: add a stabilizer to the PBS buffer solution, the stabilizer is glycine 1% of the total mass of the R1 reagent and Tween 20 accounting for 0.5% of the total mass of the R1 reagent;
[0061] Calibrator: Use the recombinant RBD protein prepared in Example 1 as an immunogen to screen out high-affinity IgG...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com