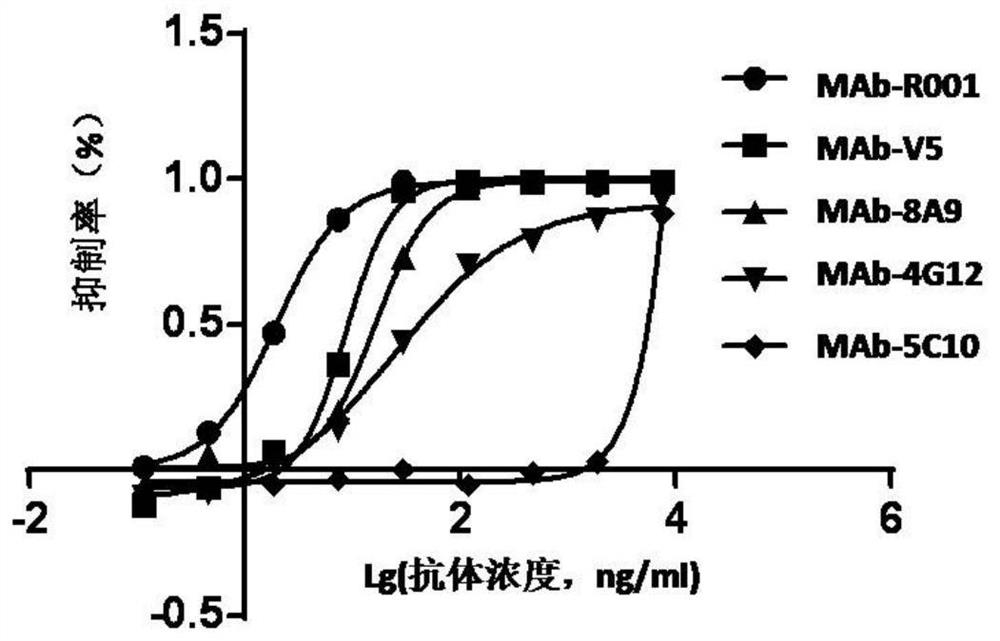
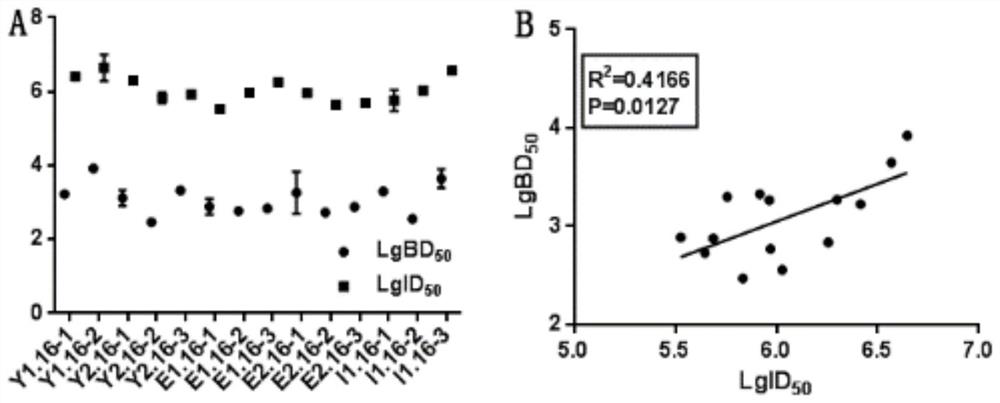
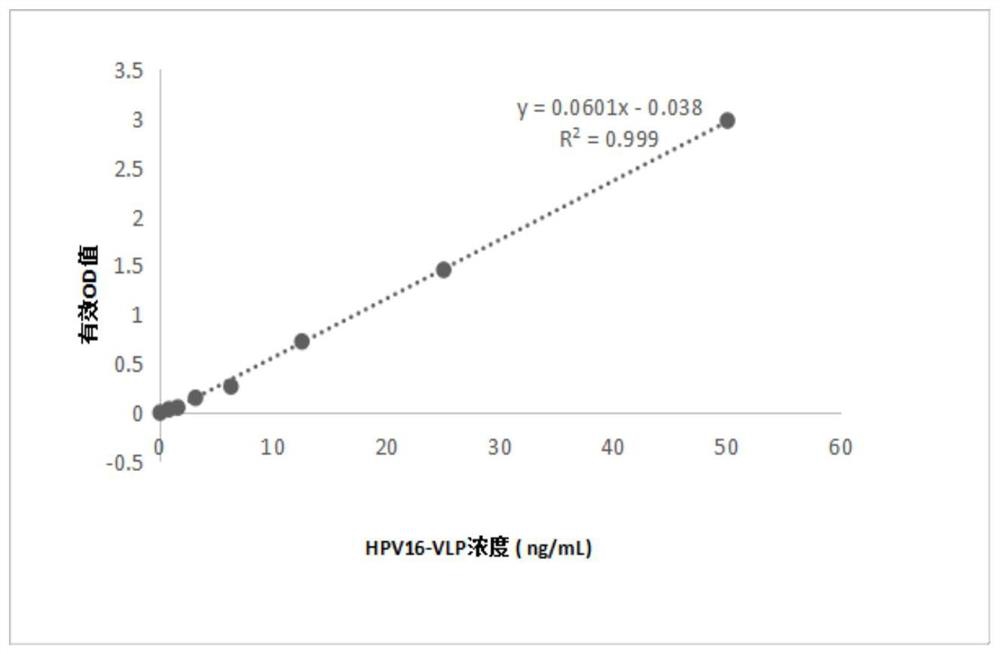
Anti-HPV16 L1 protein monoclonal antibody and detection method applying same
A technology of HPV16L1 and antibody, which is applied in the field of molecular virology and immunology, can solve the problems of large individual animal differences, long cycle, unfavorable animal welfare principles, etc., and achieve good response consistency, good neutralizing activity, and immunogenicity Evaluate the significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: Construction and screening of antibody library
[0061] 1. Construction of Antibody Library
[0062] 1.1 Animal immunity:
[0063] Get the HPV16 L1 VLP protein (SEQ ID NO: 19) expressed in the insect cell system, and emulsify it with Freund's complete adjuvant (Sigma company, product number F5881) and Freund's incomplete adjuvant (Sigma company, product number F5506) respectively to make Freund's Complete Freund's Adjuvant Immunogen and Freund's Incomplete Adjuvant Immunogen, wherein the volume ratio of HPV16 L1 (500ug / piece) to Freund's Complete Adjuvant and Freund's Incomplete Adjuvant is 1:1.
[0064]
[0065]
[0066] By way of multi-point subcutaneous injection on the back, 2-2.2 kg of Japanese big-eared white rabbits (2-2.2 kg, purchased from Beijing Baier Kangnaite Experimental Rabbit Breeding Biotechnology Development Co., Ltd.) were immunized with Freund's complete adjuvant immunogen. .
[0067] After the first immunization, the animals w...
Embodiment 2
[0087] Example 2 Construction, Production and Purification of Antibodies
[0088] Antibody construction and production:
[0089] The nucleotide sequence (SEQ ID NO:3) of the heavy chain variable region of the R001scFv antibody was spliced with the heavy chain signal peptide sequence (SEQ ID NO:11) by PCR method, and then passed Hind III and KpnI (source: Fermentas ) was digested and inserted into the pSTEP2 vector with the sequence of the rabbit IgG1 heavy chain constant region (SEQ ID NO:5) to obtain the complete expression vector of the heavy chain sequence (SEQ ID NO:9). The nucleotide sequence (SEQ ID NO:4) of the light chain variable region of the R001scFv antibody was spliced with the light chain signal peptide sequence (SEQ ID NO:12) by PCR method, and then the insertion band was digested by Hind III and BamHI The complete light chain sequence (SEQ ID NO: 10) expression vector was obtained from the pSTEP2 vector with the rabbit kappa light chain constant region (SE...
Embodiment 3
[0097] The biological identification of embodiment 3 antibody
[0098] 3.1 Identification of antibody specificity:
[0099] 3.1.1 Monoclonal antibody HPV 16 R001 has no cross-reaction with other types of HPV VLP
[0100] Using indirect ELISA method, HPV6, HPV11, HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59 complete VLP protein was diluted to 2 μg / mL with phosphate buffer at pH 7.2 , 100 μL / well was coated on the microtiter plate, the antibody to be tested was diluted to 10 ng / mL and added, and the color was developed by the secondary antibody labeled with horseradish peroxidase.
[0101] Table 2. HPV 16 R001 Antibody Specific Identification Results
[0102] coat protein OD450-blank HPV6 VLP 0.004 HPV11 VLP 0.001 HPV16 VLP 2.531 HPV18 VLP 0.000 HPV31 VLPs 0.002 HPV33 VLPs 0.001 HPV35 VLP 0.001 HPV39 VLP 0.004 HPV45 VLP 0.002 HPV51 VLPs 0.001 HPV52 VLPs 0.003 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com