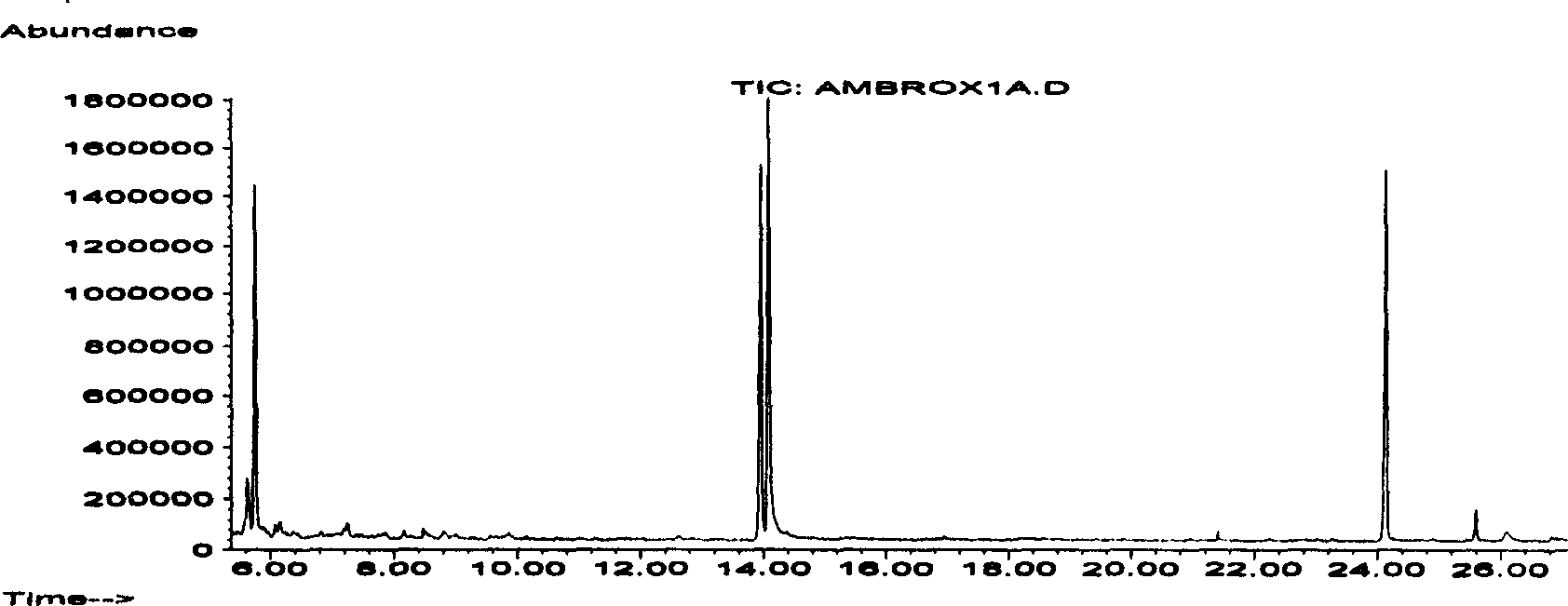
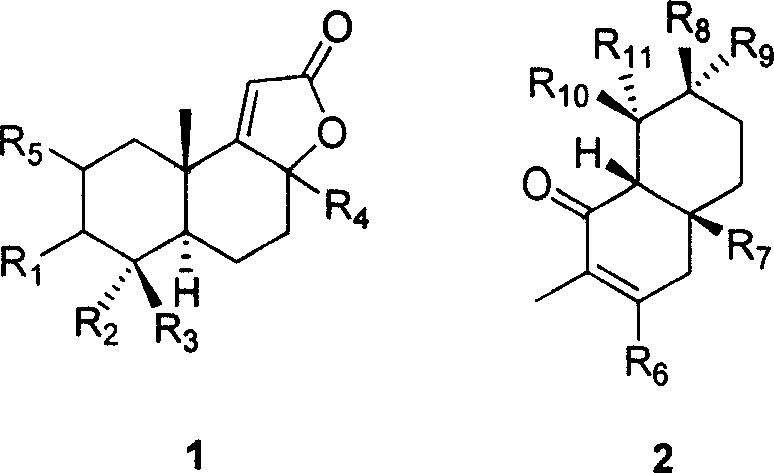
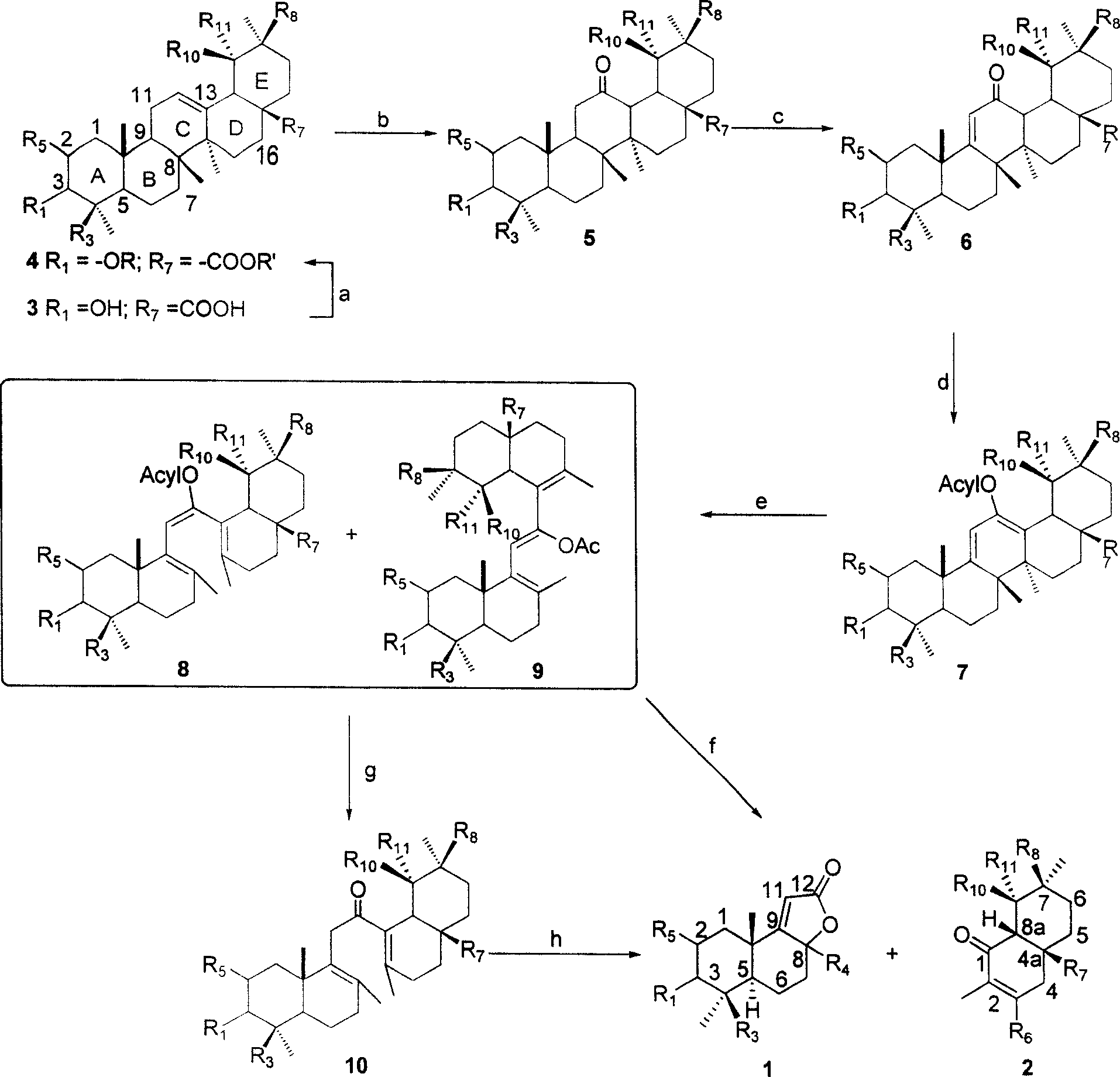
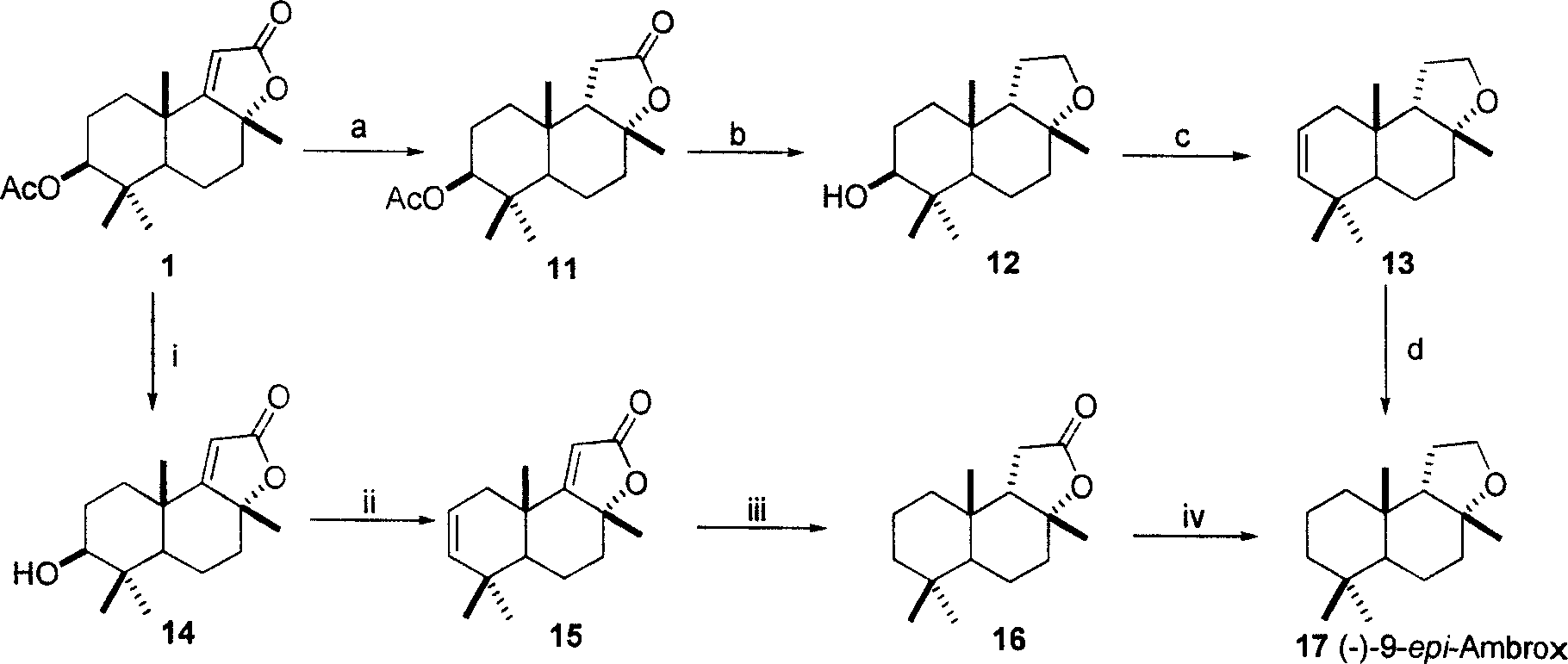
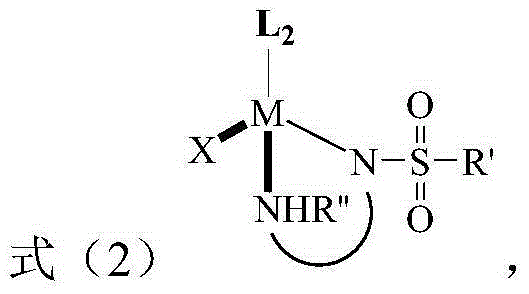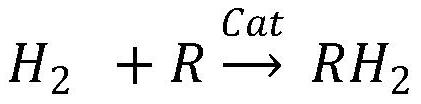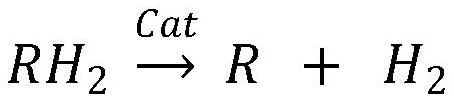Patents
Literature
46 results about "Hydronaphthalene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
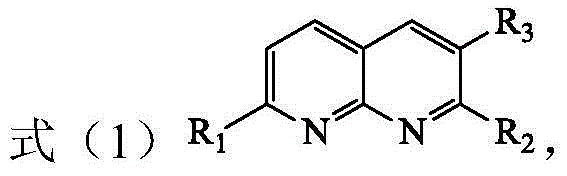
Method for preparing tetrahydro-1,5-naphthyridine compound and prepared chiral product thereof
ActiveCN104610256AAchieve selective hydrogenation reductionLow costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenCoordination complex
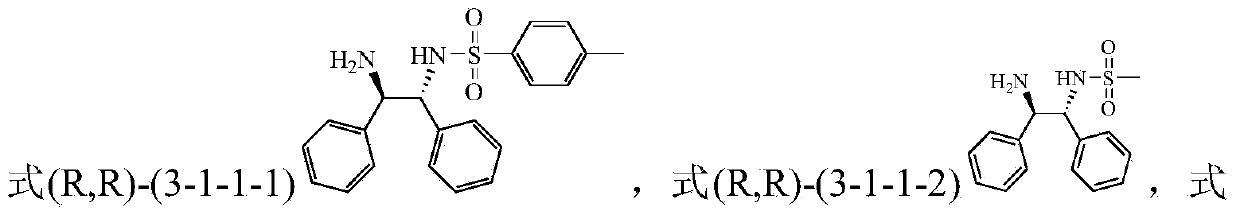
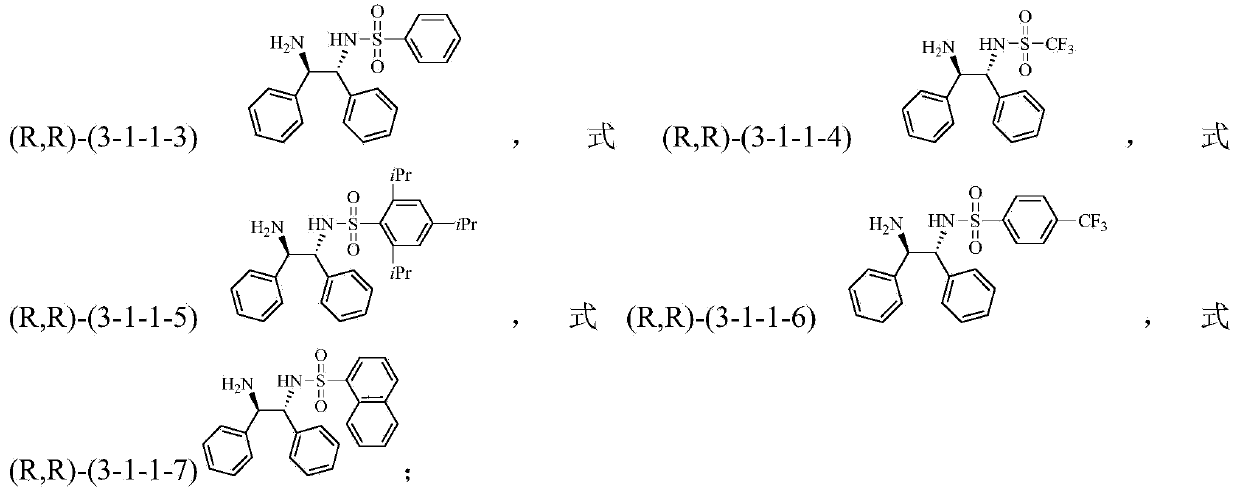
The invention discloses a method for preparing a tetrahydro-1,5-naphthyridine compound. The method comprises the following steps: carrying out an addition reaction between a compound with the structure shown as a formula (1) and hydrogen in the presence of a chiral catalyst, wherein the chiral catalyst is a coordination complex with a structure shown as a formula (2). The invention also provides a chiral product of the tetrahydro-1,5-naphthyridine compound prepared by the method. The proper compound with the structure shown as the formula (1) is selected as a substrate, the proper coordination complex with the structure shown as the formula (2) is selected as the chiral catalyst, and selective hydrogenation reduction of the 1,5-naphthyridine compound with the structure shown as the formula (1) is realized by adopting hydrogen, so that the chiral product of the tetrahydro-1,5-naphthyridine compound is prepared at low cost. The chiral product of the tetrahydro-1,5-naphthyridine compound prepared by the invention can serve as a structure block of bioactive compounds and chiral drugs.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Preparation method of tetrahydro 1, 8-naphthyridine compound and chiral product prepared by adopting preparation method
ActiveCN105111208AAchieve selective hydrogenation reductionLow costOrganic chemistryHydrogenCoordination complex
The invention discloses a preparation method of a tetrahydro 1, 8-naphthyridine compound. The preparation method comprises the following steps: under the existence of a chiral catalyst, enabling a compound with the structure shown in the formula (1) (in the description) and hydrogen to be subjected to addition reaction, wherein the chiral catalyst is a coordination compound with the structure shown in the formula (2) (in the description). The invention further provides a chiral product of the tetrahydro 1, 8-naphthyridine compound, prepared through the preparation method. According to the invention, the proper compound with the structure shown in the formula (1) (in the description) is used as a substrate, and the proper coordination compound with the structure shown in the formula (2) (in the description) is used as the chiral catalyst to perform selective hydrogenation reduction on 1, 8-naphthyridine compound with the structure shown in the formula (1) by adopting hydrogen, so that the chiral product of the tetrahydro 1, 8-naphthyridine compound is prepared with low cost. The chiral product of the tetrahydro 1, 8-naphthyridine compound can be used as a biologically active compound and a structural building block of a chiral drug.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Oxalate for preparing ambrox or releasing ambrox in cigarette smoke and its use
An application of the oxalate in preparing norambrether or releasing the norambrether to the smoke of cigaretter is disclosed. Said oxalate may be decahydro-2-hydroxy-2,5,5,8a-tetramethyl-naphthal eneethanol or 2-hydroxy-2,5,5,9-tetramethyl decalyl ethanol. It can be proportionally added to cigarette, or can be decomposed to obtain norambrether.
Owner:CHINA TOBACCO HUNAN INDAL CORP
Polysubstitution hydrogenated naphthalene compounds, producing method and uses of the same
The present invention belongs to the field of organic chemistry and relates to a multi-substituted hydrogenated naphthalene compound, the preparation methods and uses. Specifically, the present invention relates to a chiral multi-substituted ten-hydrogen and / or eight-hydrogen naphthalene compound, the synthetic method and uses. The present invention aims to provide a chiral multi-substituted ten-hydrogen and / or eight-hydrogen naphthalene compound; the oleanane-type or usu-type five-ring triterpenoid compound is used as a raw material for preparing the compound. The multi-substituted ten-hydrogen and / or eight-hydrogen naphthalene compound of the present invention can be used for synthesis of drugs or spices containing multi-hydrogen naphthalene fragments and the analogues. The method of the present invention is simple and easy, low in cost, high in production rate, and can realize industrialization.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Preparation method of industrial musk tonalide
InactiveCN102050715AExtended reaction timeHigh selectivityCarbonyl compound preparation by condensationP-isopropyltolueneDistillation
The invention relates to a preparation method of industrial musk tonalide, which comprises the following steps: carrying out Friedel-Crafts alkylation by using p-isopropyl toluene and 2,3-dimethyl-1-butene as raw materials and using tertiary butyl chloride as a hydrogen absorption agent to synthesize an intermediate 1,1,3,4,4,6-hexamethyl tetrahydronaphthalene; and in a dichloromethane solvent, carrying out Friedel-Crafts alkylation on the intermediate used as the raw material and acetyl chloride, thereby obtaining the product musk tonalide (7-acetyl-1,1,3,4,4,6-hexamethyl tetrahydronaphthalene, AHMT). Compared with the existing method and technology, the method provided by the invention is simple to operate, has the characteristics of high reaction speed, high yield and the like, and realizes industrial production; and the product can be simply purified by thermally dissolving out pigments by using anhydrous alcohol without distillation or rectification.
Owner:HENAN UNIV OF SCI & TECH
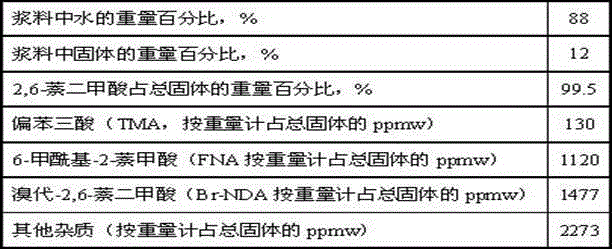
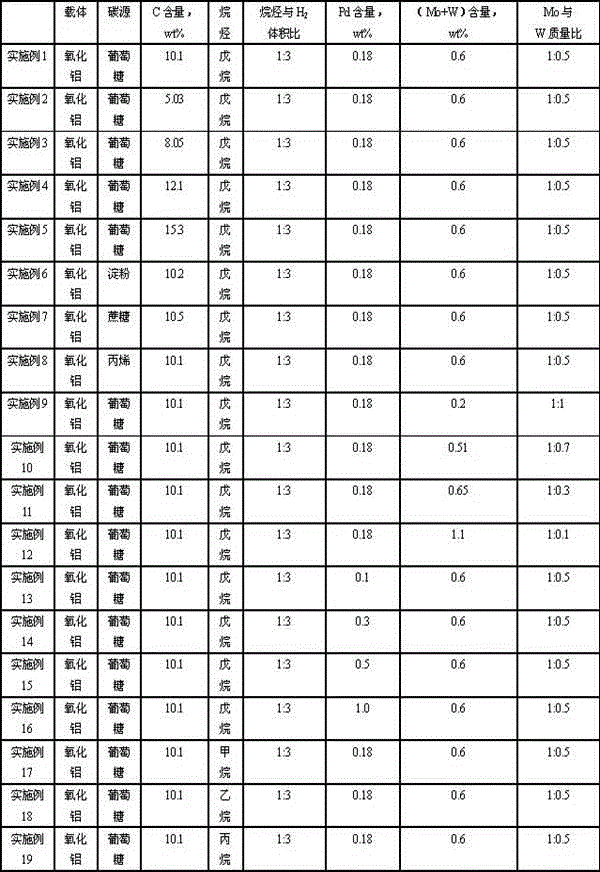
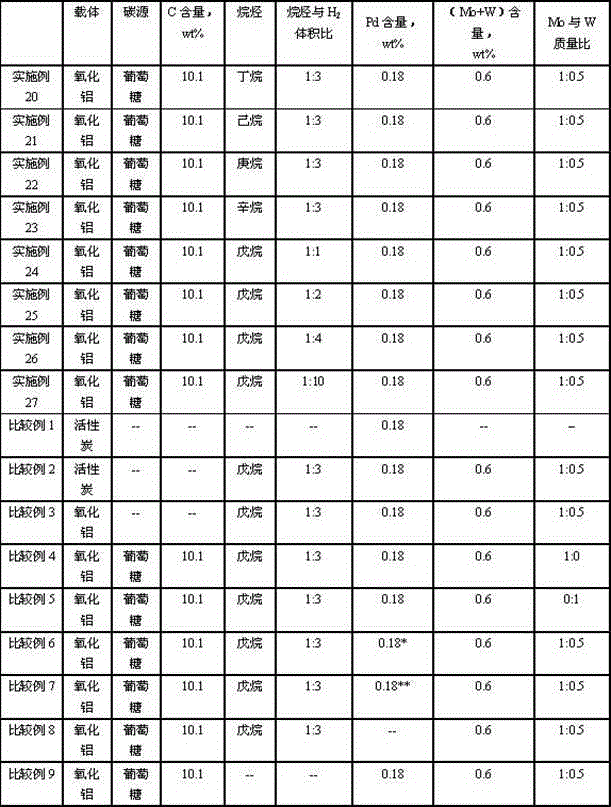
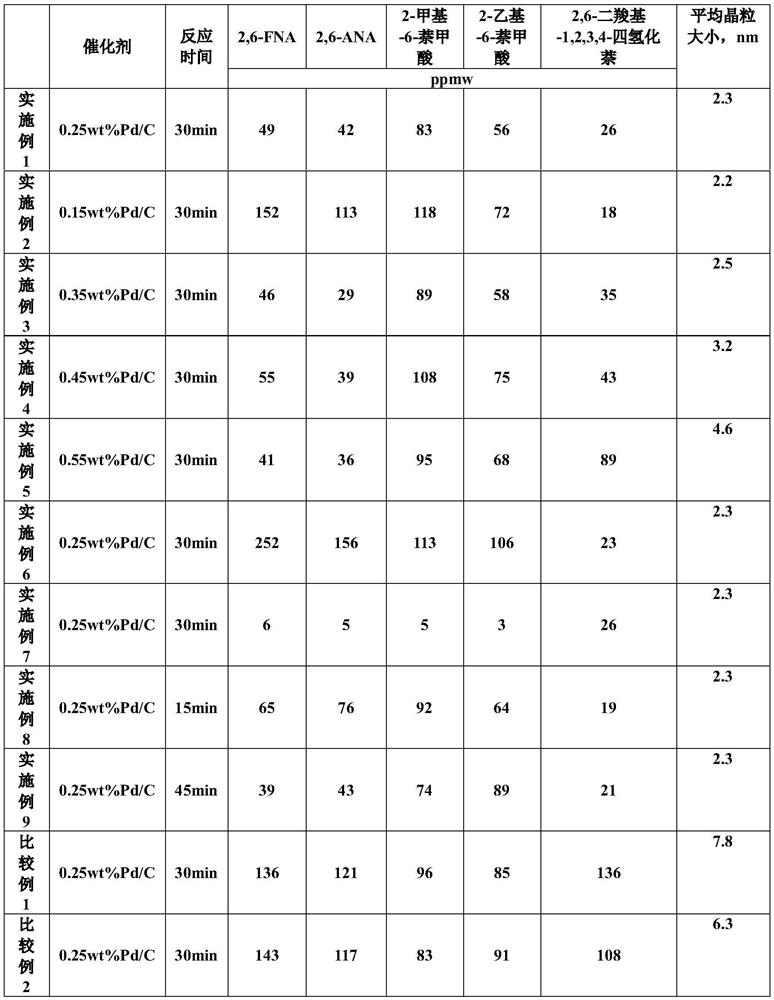
Catalyst for hydrogenating and purifying crude 2,6-naphthalic acid and preparation method of catalyst
ActiveCN104549264AExcessive hydrogenation is not easy to occurHigh activityOrganic compound preparationCarboxylic compound preparationPtru catalystCarboxyl radical
The invention relates to a catalyst for hydrogenating and purifying crude 2,6-naphthalic acid and a preparation method of the catalyst, aiming at solving the problems that the crude 2,6-naphthalic acid can be excessively hydrogenated to generate 2,6-dicarboxyl tetrahydronaphthalene when being subjected to hydrogenation and purification in the prior art. According to the catalyst, carbon-covered aluminum oxide is used as a carrier, Pd is used as an active component, and Mo and W are used as catalyst promoters; the content of carbon in the carbon-covered aluminum oxide is 5-15wt%, the Pd content of the catalyst is 0.1-1wt%, and the Mo content is 0.1-1wt%; and the mass ratio of Mo to W is 1: (0.1-1). The catalyst can solve the problem well and can be used for industrial production of hydrogenation and purification of the crude 2,6-naphthalic acid.
Owner:CHINA PETROLEUM & CHEM CORP +1
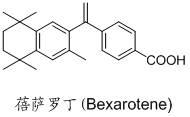
Bexarotene hydroximic acid as well as preparation method and application thereof
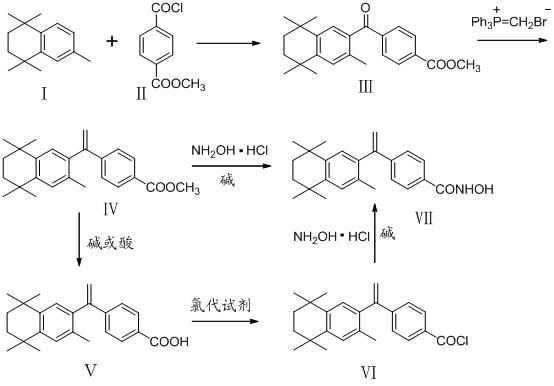
InactiveCN102503857AHas anti-tumor effectEasy to prepareOrganic chemistryAntineoplastic agentsHydroxizinumCancer cell
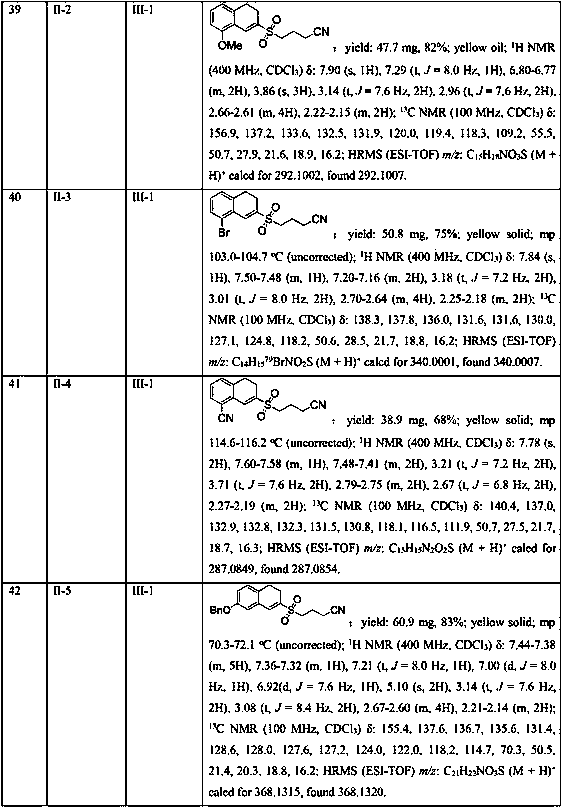
The invention belongs to the technical field of medicine and discloses bexarotene hydroximic acid as well as a preparation method and application thereof. The formation of the compound is as shown in the picture and the objective compound is prepared mainly by taking 1,1,4,4,6- pentamethyl-1,2,3,4-tetrahydronaphthalene as the initial material through the steps of Friedel-Crafts acylation, Wittig reaction and condensation reaction. The method is simple in operation, convenient in post-processing and high in yield. The objective compound has good inhibitory action on various cancer cells by the multiple action mechanisms of bonding the retinoic acid X receptors and restraining the histone deacetyltransferases, and the anti-cancer activity of the bexarotene hydroximic acid is obviously better than that of bexarotene. Therefore, the bexarotene hydroximic acid can be applied to treatment of cancers.
Owner:SHENYANG PHARMA UNIVERSITY
Separating and purifying device and process for heavy-component residual liquid of hydrogenation process of coking crude benzene
ActiveCN101982214ALow boiling pointChanging the relative volatilityDistillation purification/separationVacuum distillation separationBenzeneTetralin
The invention discloses a separating and purifying device and process for heavy component residual liquid of a hydrogenation process of coking crude benzene, belonging to the technical field of fractioning separation. The separating and purifying device of the invention comprises a tower kettle, a batch fractioning tower, a tower top fraction storage tank and a vacuum pump, wherein the batch fractioning tower is connected with the tower kettle; a condenser is arranged at the tower top of the batch fractionating tower; and the vacuum pump is respectively connected with the condenser and the tower top fraction storage tank through a buffer tank. The process comprises the following steps: when the tower top pressure is 10.0-15.0kPa, and the tower top temperature is 115.0-120.0 DEG C, collecting indan from the tower top by a reflux ratio of 4-5; after the indan is collected, when the tower top pressure is 10.0-15.0kPa, and the tower top temperature is 125.0-130.0 DEG C, collecting tetraline from the tower top by the reflux ratio of 8-10; and finally when the tower top pressure is 10.0-15.0kPa, and the tower top temperature is 155.0-160.0 DEG C, collecting naphthalene from the tower top by a reflux ratio of 4-5. The purity of the indan is 99.0wt%, the purity of the tetraline is 99.5wt%, and the purity of the naphthalene is 98.0wt%.
Owner:XINGTAI RISUN COAL CHEM IND
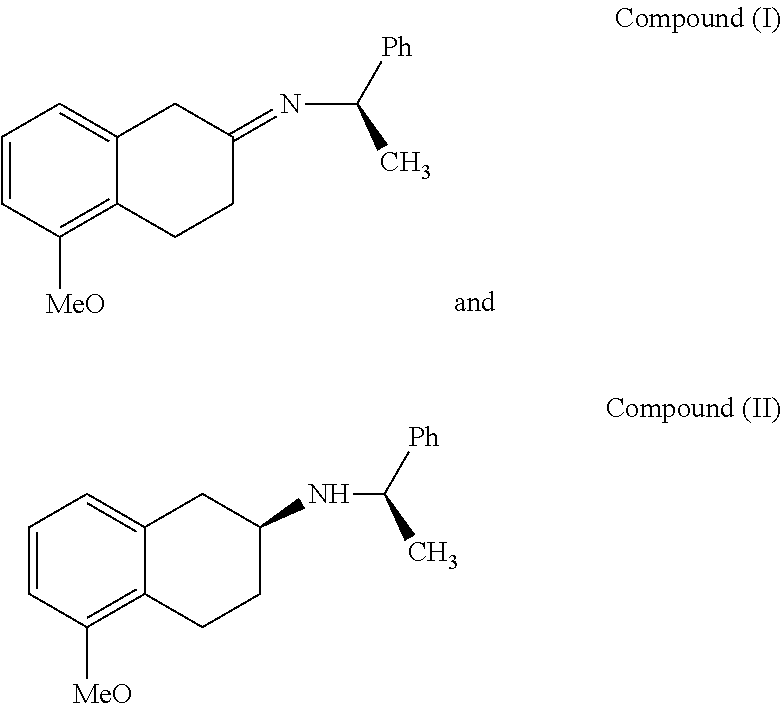
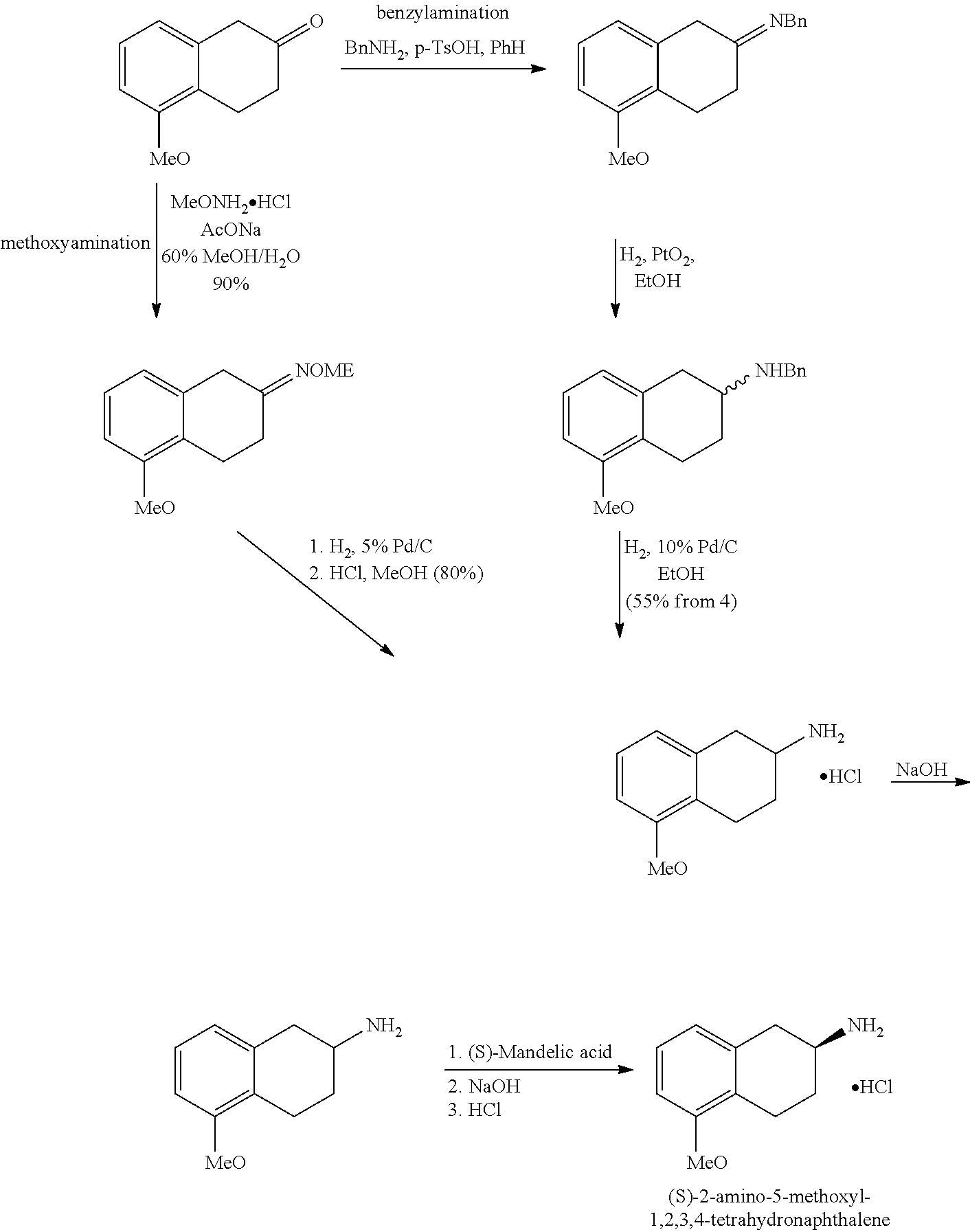
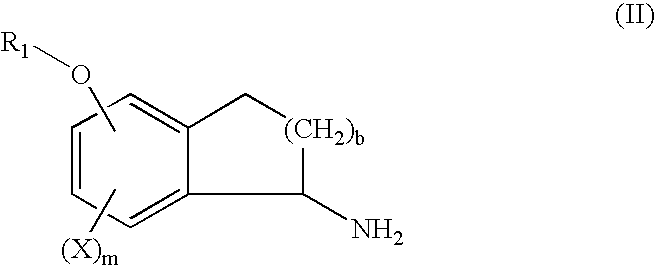
Method of Preparing (S)-2-amino-5-Methoxytetralin Hydrochloride
ActiveUS20140046095A1Increase productionLow yieldIsocyanic acid derivatives preparationOrganic compound preparationTetralin2-Tetralone
A method of preparing (S)-2-amino-5-methoxytetralin hydrochloride[(S)-2-amino-5-methoxyl-1,2,3,4-tetrahydronaphthalene hydrochloride], comprising the steps of: (1) producing a compound (I) by addition-elimination reaction of 5-methoxy-2-tetralone and R-(+)-a-phenylethylamine; (2) producing a compound (II) by reduction reaction of the compound (I) with a reducing agent; and (3) producing a compound (II) hydrochloride by reacting the compound (II) with a salt-forming agent, then carrying out reduction reaction with a palladium-carbon catalyst to produce (S)-2-amino-5-methoxytetralin hydrochloride. The method can significantly increase the yield of (S)-2-amino-5-methoxytetralin hydrochloride with short synthetic path, low preparation cost and less pollution, which is environmentally friendly and is suitable for medical industrialized production. The structural formulae of the compound (I) and the compound (II) are:resepectively.
Owner:ANHUI QINGYUN PHARMA & CHEM
Process for the synthesis of indanylamine or aminotetralin derivatives and novel intermediates
InactiveUS20060052639A1Easy to handleOrganic compound preparationCarboxylic acid amides preparationTetraloneHydrogen
A process for preparing indanylamine and aminotetralin derivatives from indanone or tetralone oximes by acylating the oximes with an organic anhydride, followed by catalytic hydrogenation in the presence of an organic anhydride with subsequent hydrolysis is described. The process is commercially feasible providing indanylamine and aminotetralin derivatives in high yield that are useful as intermediates in the production of therapeutically active compounds. Also described are novel intermediates, 1-indanone O-acetyl oximes and 1-tetralone O-acetyl oximes.
Owner:TEVA PHARMA IND LTD
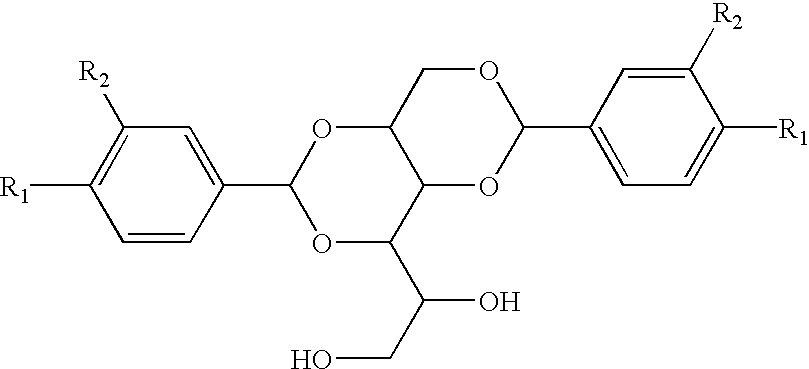
Novel asymmetric substituted benzaldehyde alditol derivatives and compositions and articles containing same
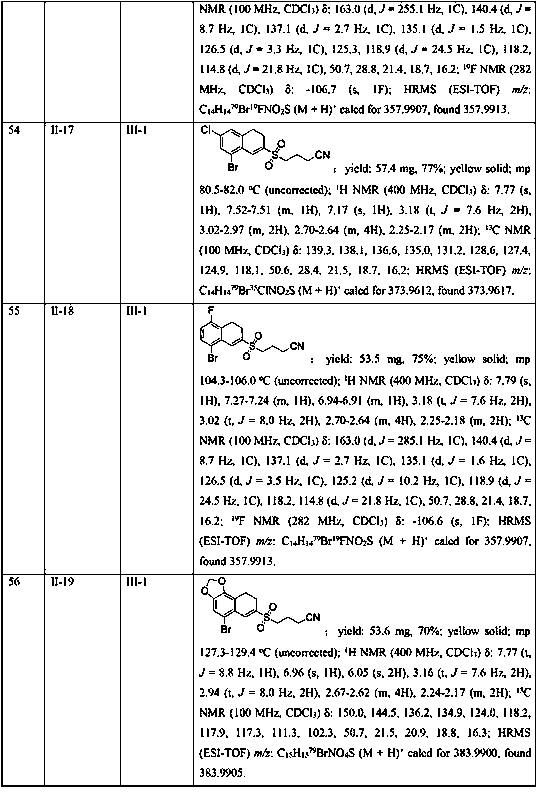
Plastic additives which are useful as nucleating agents and which are especially useful for improving the optical properties of polymeric materials are provided. More particularly, this invention relates to certain asymmetric DBS compounds comprising specific pendant groups, such as C1-C6 alkyl, C1-C6 alkoxy, phenyl, naphthyl, or substituted phenyl, or pendant groups combined to cyclic moities, such as cyclopentyl (and thus to form indan with the benzylidene), cyclohexyl (to form tetralin), and methylenedioxy (as the combination of two available sites on the pertinent ring system). Such compounds may be added to or incorporated within polymer compositions which may then be utilized within, as merely examples, food or cosmetic containers and packaging. These inventive asymmetric benzylidene sorbitol acetals are also useful as gelling agents for water and organic solvents, particularly those used in the preparation of antiperspirant gel sticks. Preferably one of the benzylidene moieties is 3,4-disubstituted, with most preferably methyl groups in the 3 and 4 positions.
Owner:MILLIKEN & CO
7-substitution-1-hero hydrogenated-2-ketone and ramification as well as its synthesizing method and usage
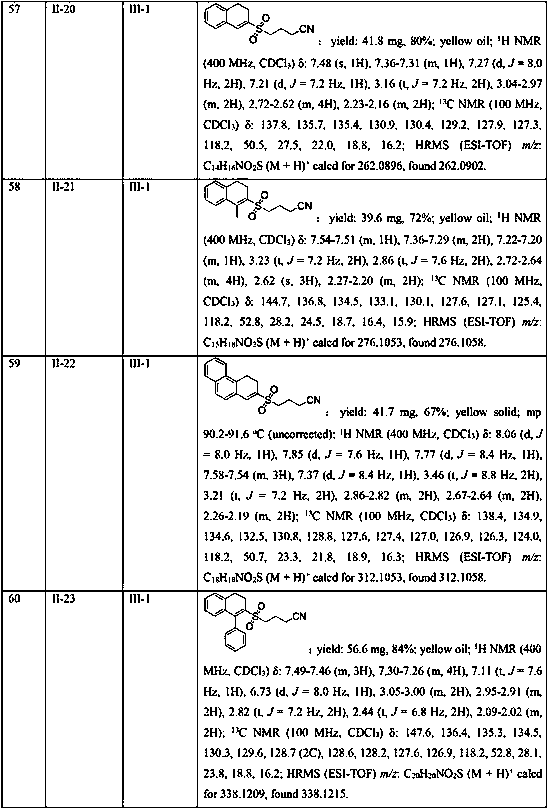
A compound 7-substitutent-1-naphthalene heterohydride-2-one and its derivatives, which has high activity to resist HBV, its preparing process, and its application in preparing medicine to treat hepatitis B are disclosed. Its advantage is higher suppression rate of HBsAg.
Owner:FUDAN UNIV
Synthesis method of 2-cyanoalkylsulfonyl 3,4-dihydronaphthalene compound
ActiveCN111039737AHigh yieldConvenient sourceSulfonyl/sulfinyl group formation/introductionOrganic compound preparationAcyl groupOxime
The invention discloses a synthesis strategy for constructing a 2-cyanoalkyl sulfonyl substituted 3,4-dihydronaphthalene compound by taking MCPs, a cyclobutanone oxime ester compound and K2S2O5 as rawmaterials through visible light catalysis, wherein two carbon-carbon sigma bonds are broken in a one-pot reaction to form a carbon-carbon bond and two carbon-sulfur bonds. According to the invention,cyanoalkyl free radicals are formed to capture SO2, and subsequently sulfonyl free radicals are formed to carry out ring-opening and cyclizing on MCPs; and the synthesis method disclosed by the invention has the advantages of mild reaction conditions, simplicity, high efficiency, easily available raw material sources, wide substrate application range and high target product yield.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
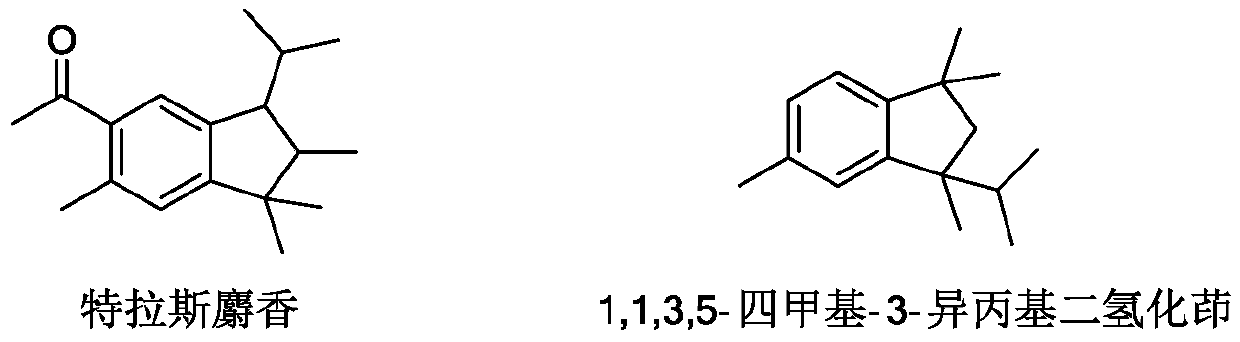
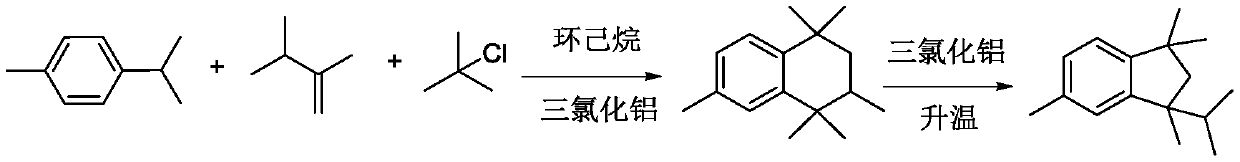
Synthesis method of 1, 1, 3, 5-tetramethyl-3-isopropyl hydrindene
PendingCN110698316AMake it easier to getHigh yieldHydrocarbon by isomerisationCatalystsIsomerizationMeth-
The invention relates to a synthetic method of 1, 1, 3, 5-tetramethyl-3-isopropyl hydrindene. The synthetic method specifically comprises the following steps: S1, uniformly mixing p-cymene, chlorotert-butane and 2, 3-dimethyl-1-butene, dropwise adding an obtained mixed solution into a reaction kettle filled with cyclohexane and aluminum trichloride, stirring for reaction, washing with water and alkali for elution, and rectifying to remove p-cymene, so as to obtain a 1, 1, 3, 4, 4, 6-hexamethyltetrahydronaphthalene crude product; S2, transferring the 1, 1, 3, 4, 4, 6-hexamethyl tetrahydronaphthalene crude product obtained in the step S1 into an isomerization kettle, then adding dichloroethane and aluminum trichloride, and carrying out stirring, heating and reflux reaction; S3, washing a product obtained after the isomerization reaction with water and alkali until the product is neutral, desolventizing to remove dichloroethane, transferring an obtained desolventized 1, 1, 3, 5-tetramethyl-3-isopropyl hydrindene crude product into a crystallization kettle, adding a crystallization solvent, and recrystallizing for multiple times to obtain the 1, 1, 3, 5-tetramethyl-3-isopropyl hydrindene. According to the synthetic method of the 1, 1, 3, 5-tetramethyl-3-isopropyl hydrindene, provided by the invention, the yield of a target product is improved, and the purification difficulty is reduced.
Owner:TIANMEN DEYUAN CHEM TECH CO LTD
Process for the synthesis of indanylamine or aminotetralin derivatives and novel intermediates
InactiveUS7262326B2Organic compound preparationCarboxylic acid amides preparationTetraloneHydrolysis
A process for preparing indanylamine and aminotetralin derivatives from indanone or tetralone oximes by acylating the oximes with an organic anhydride, followed by catalytic hydrogenation in the presence of an organic anhydride with subsequent hydrolysis is described. The process is commercially feasible providing indanylamine and aminotetralin derivatives in high yield that are useful as intermediates in the production of therapeutically active compounds. Also described are novel intermediates, 1-indanone O-acetyl oximes and 1-tetralone O-acetyl oximes.
Owner:TEVA PHARMA IND LTD
Process for preparation of 2,6-dialkyltetralin
InactiveUS7525001B2Organic compound preparationPreparation by hydrogenationBiochemical engineeringProcess engineering
The present invention provides a novel process for highly selective preparation of 2,6-dialkyltetralin, a key precursor for 2,6-dimethylnaphthalene (2,6-DMN), which does not require an extra step for purifying various isomers obtained from the conventional processes for 2,6-DMN. The present invention is advantageous to improve the synthetic yield, to simplify the operation and thus to reduce the production cost, since different starting materials and different pathways are exploited and thus the additional steps are not necessary.
Owner:SEOUL NAT UNIV R&DB FOUND
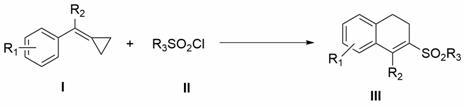
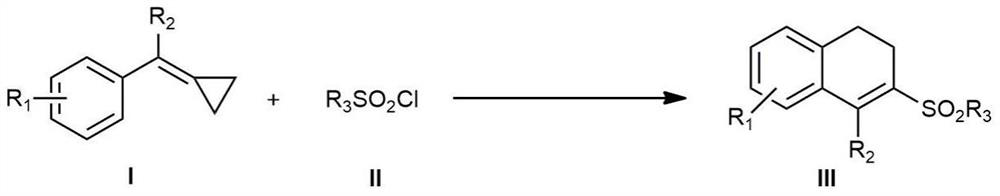
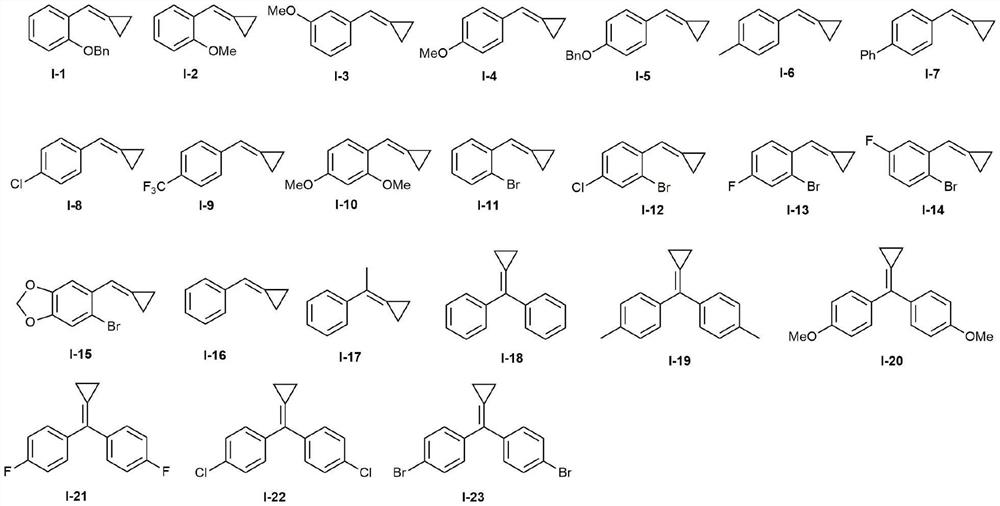
A kind of photocatalytic preparation method of 3-sulfonyl-1,2-dihydronaphthalene compound
InactiveCN109705001BConvenient sourceMild reaction conditionsOrganic chemistryOrganic compound preparationSulfonyl chlorideMeth-
The invention discloses a method for photocatalytically preparing 3-sulfonyl-1,2-dihydronaphthalene compounds. The method employs a methylene cyclopropane compound and sulfonyl chloride as initial rawmaterials, then in the presence of a photocatalyst and visible light, various 3-sulfonyl-1,2-dihydronaphthalene compounds are synthesized through C=C sulfonylation, C-C [sigma]-bond rupture and intramolecular cyclization. The method employs accessible raw materials and mild reaction conditions, is simple in operation, is wide in substrate adaptation range and is high in yield.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Hydrofining method of crude 2, 6-naphthalic acid
PendingCN114426480AOrganic compound preparationCatalyst activation/preparationPtru catalystCarboxyl radical
The invention relates to a hydrofining method of crude 2, 6-naphthalic acid, and mainly solves the problem that in the prior art, during hydrofining of crude 2, 6-naphthalic acid, a large amount of impurity 2, 6-dicarboxyl-1, 2, 3, 4-tetrahydronaphthalene is generated. The crude 2, 6-naphthalic acid hydrofining method comprises the following steps: in the presence of a hydrofining catalyst, water is taken as a solvent, crude 2, 6-naphthalic acid reacts with hydrogen to obtain refined 2, 6-naphthalic acid, the catalyst comprises a carrier and a noble metal loaded on the carrier, the noble metal comprises palladium, and the noble metal comprises palladium. The average grain size of palladium is 2-5nm through X-ray diffraction analysis, so that the problem is well solved, and the method can be used for producing refined 2, 6-naphthalic acid.
Owner:CHINA PETROLEUM & CHEM CORP +1
Oxygen-absorbing resin composition
ActiveUS10150107B2Improve performanceExcellent toneGas treatmentOther chemical processesPolyesterPolymer science
Oxygen-absorbing resin composition comprising a polyester containing a constitutional unit (a) comprising tetralin ring structure, for example, an alkyl tetralin dicarboxylate, wherein the alkyl component has, for example, from 2 to 6 carbon atoms, and a constitutional unit (b) derived from a polyfunctional compound, selected from glycerin, trimethylol propane, pentaerythritol, trimellitic acid, trimellitic acid anhydride, pyromellitic acid, and pyromellitic acid anhydride, and a transition metal catalyst.
Owner:MITSUBISHI GAS CHEM CO INC
Method of synthesizing alkyl methyl tetra hydronaphthalene using alkyl benzene and isopentadiene as raw material
InactiveCN1618778AEasy to manufactureSimple processHydrocarbon from saturated and unsaturated hydrocarbon additionAlkyl transferMolecular sieve
A process for synthesizing naphthalene alkylmethyltetrahydride from alkylbenzene and isopentandiene includes alkylation reaction between C1-C5 alkylbenzene and isopentandiene under the existance of Lewis acid or Y-type molecular sieve to obtain pentenylalkyl benzene, and cyclizing reaction under existance of Y-type molecular sieve. Its advantages are simple process and low cost.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD +1
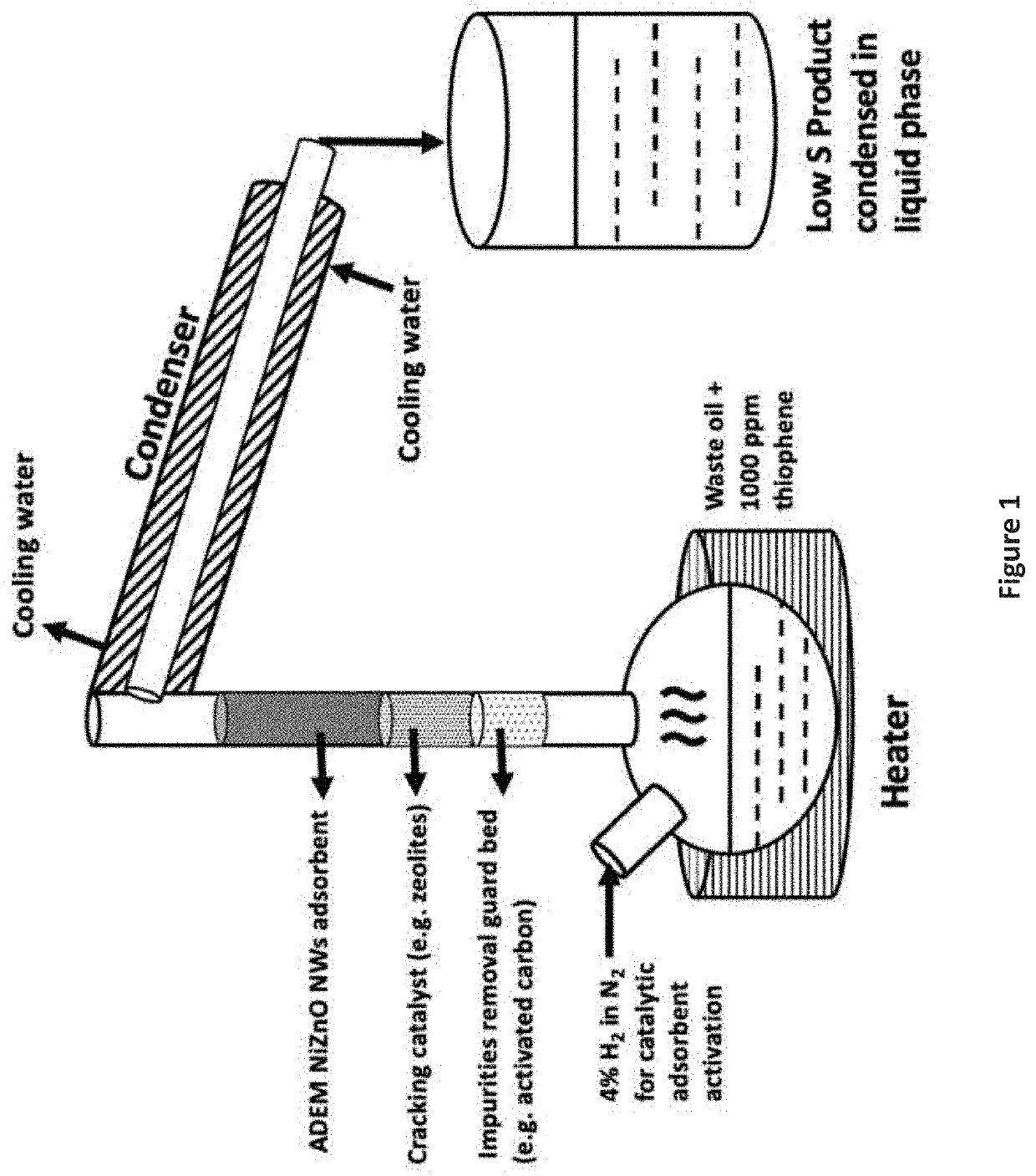
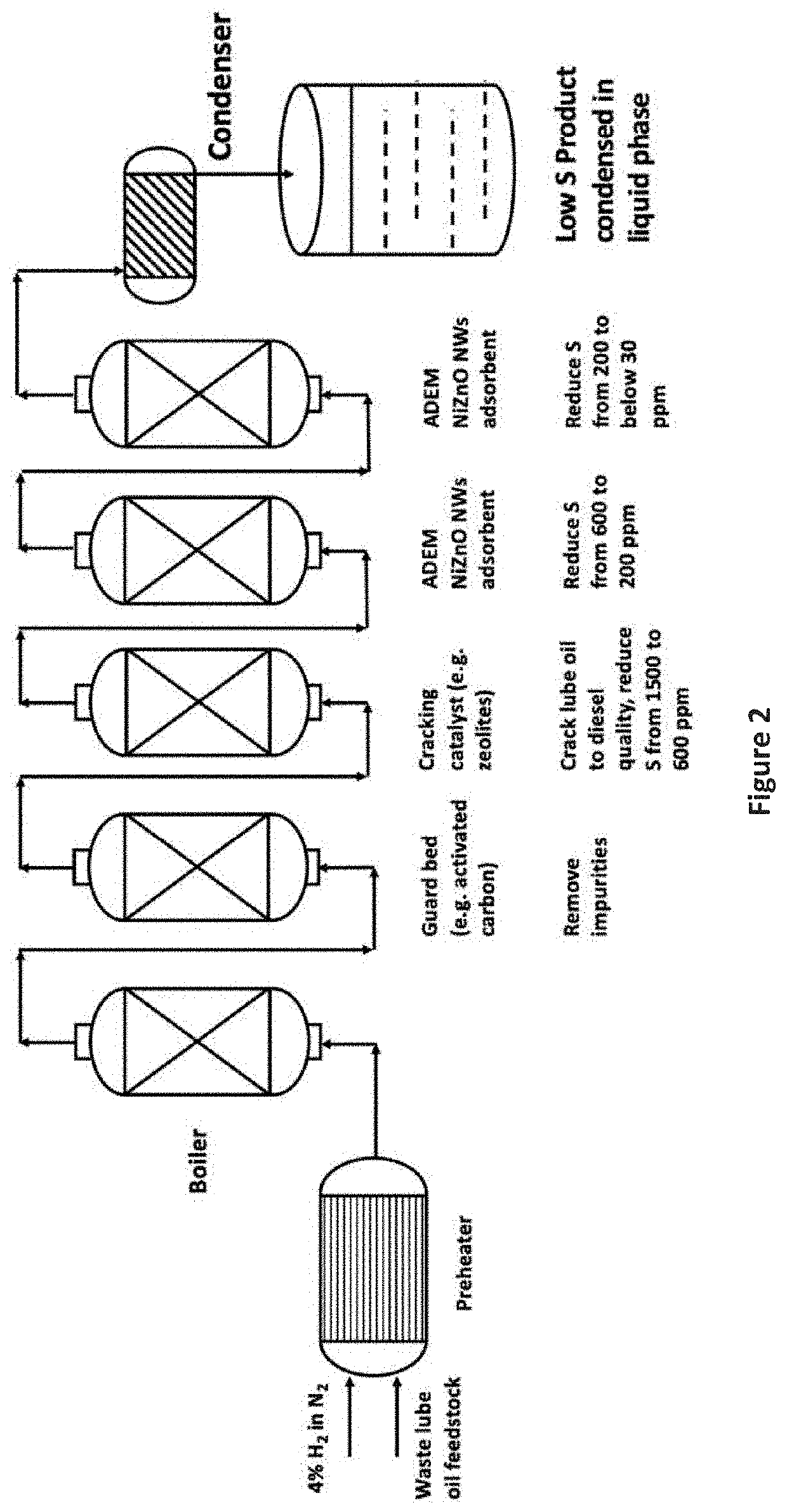
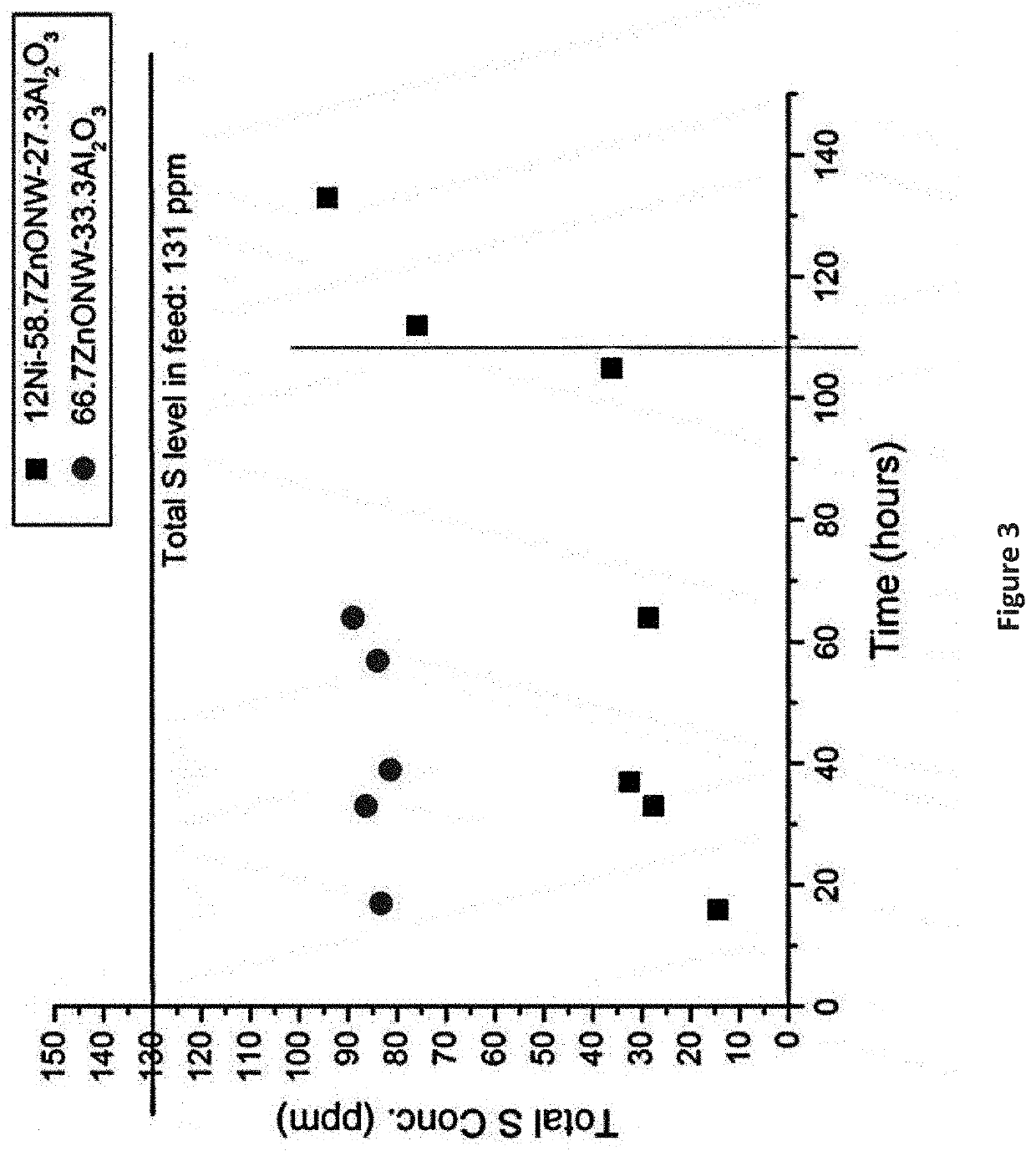
Desulfurization and Sulfur Tolerant Hydrogenation Processes of Hydrocarbon Feedstocks
PendingUS20220184580A1Reduce the concentration of sulfurRefining with metalsLiquid degasification with auxillary substancesPtru catalystTetralin
The present invention is a method for removing sulfur from liquid hydrocarbon feedstocks and for performing hydrogenation reactions in sulfur-contaminated feedstocks, including the hydrogenation of naphthalene in the presence of sulfur compounds, using catalysts or adsorbents comprising metal oxide nanowires decorated with reduced catalytically-active metal particles. In a preferred embodiment, the adsorbent comprises zinc oxide nanowires decorated with catalytically-active metals selected from nickel, cobalt, molybdenum, platinum, palladium, copper, oxides thereof, alloys thereof, and combinations thereof. In some embodiments, the sulfur is removed through a desulfurization process without an external hydrogen supply. The process is effective for the removal of sulfur from diesel fuels and liquid fuel streams, and for deep desulfurization of natural gas streams. The process is also effective for the selective hydrogenation of naphthalene to tetralin in the presence of sulfur compounds.
Owner:ADVANCED ENERGY MATERIALS LLC
Process for the preparation of diisocyanates by phosgenation of diamine suspensions
ActiveCN108147980BIsocyanic acid derivatives preparationOrganic compound preparationPolymer scienceToluidine
The present invention relates to a process for the preparation of diisocyanates by phosgenation of diamine suspensions. In particular, a subject of the invention is a process for the preparation of organic diisocyanates by reacting the corresponding diamines with phosgene in an inert solvent. A high-melting diamine selected from the group consisting of 1,5-naphthalene diamine, tetrahydronaphthalene diamine, 1,4-phenylene diamine, durene diamine and di-o-toluidine diamine is used as diamine. According to the invention, a suspension of said diamine is prepared in an inert solvent, using a dynamic mixing device selected from a disc disperser and a rotor-stator-system, and the suspension obtained is phosgenated.
Owner:COVESTRO DEUTSCHLAND AG
A kind of synthetic method of alkoxy substituted 3,4-dihydronaphthalene compounds
InactiveCN109776297BConvenient sourceLow priceCarboxylic acid nitrile preparationOrganic compound preparationRotary evaporatorPtru catalyst
The invention discloses a method for synthesizing 2-(2-oxopropyl)-3,4-dihydronaphthalene compounds and 1-(3-oxobutyl)-3,4-dihydronaphthalene compounds . Add the methenylcyclopropane derivatives of formula II, silver catalyst and oxidizing agent successively to the Schlenk sealed tube reactor, then add the ketone compound shown in formula III, replace the air in the reactor with argon or nitrogen, replace After 3-5 times, heat the reaction in an oil bath at 60-120° C. for 12-36 hours under argon or nitrogen atmosphere. After the reaction was complete, the solvent was evaporated to dryness with a rotary evaporator, and the residue was separated and purified with a chromatographic column to obtain the target product represented by formula I.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Preparation method of industrial musk tonalide
InactiveCN102050715BExtended reaction timeHigh selectivityCarbonyl compound preparation by condensationP-isopropyltolueneDistillation
The invention relates to a preparation method of industrial musk tonalide, which comprises the following steps: carrying out Friedel-Crafts alkylation by using p-isopropyl toluene and 2,3-dimethyl-1-butene as raw materials and using tertiary butyl chloride as a hydrogen absorption agent to synthesize an intermediate 1,1,3,4,4,6-hexamethyl tetrahydronaphthalene; and in a dichloromethane solvent, carrying out Friedel-Crafts alkylation on the intermediate used as the raw material and acetyl chloride, thereby obtaining the product musk tonalide (7-acetyl-1,1,3,4,4,6-hexamethyl tetrahydronaphthalene, AHMT). Compared with the existing method and technology, the method provided by the invention is simple to operate, has the characteristics of high reaction speed, high yield and the like, and realizes industrial production; and the product can be simply purified by thermally dissolving out pigments by using anhydrous alcohol without distillation or rectification.
Owner:HENAN UNIV OF SCI & TECH
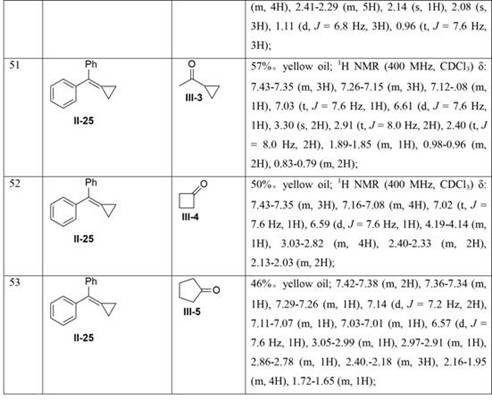
Preparation method of tetrahydronaphthalene benzamide key intermediate
PendingCN114105908AShort reaction timeImprove time and efficiencyOrganic chemistryMethylanilineCompound a
The invention discloses a preparation method of a tetrahydronaphthalene benzamide key intermediate. The preparation method comprises the following steps: adding a compound a into a reaction container, adding a reaction solvent for dissolving, and adding 3-iodine-4-methylaniline while controlling the temperature at 5-45 DEG C; vacuumizing the obtained reaction system, introducing nitrogen for protection, controlling the temperature at 15-25 DEG C, slowly dropwise adding a catalyst, heating the reaction system to 80-110 DEG C after dropwise adding, and reacting for 3-4 hours to obtain a reaction mixture; and adding an extraction system into the obtained reaction mixture for extraction, separating and taking an organic phase, washing, drying and evaporating or concentrating and crystallizing to obtain the tetrahydronaphthalene benzamide key intermediate. According to the method disclosed by the invention, the reaction time is greatly shortened, the yield is improved, the use of a catalyst is reduced, byproducts in the reaction are reduced, and the cost is greatly saved.
Owner:LIAONING UNIVERSITY
A method for efficiently synthesizing (1r,2s)-1,2-dihydronaphthalene derivatives
InactiveCN108863844BLow costImprove efficiencyCarbamic acid derivatives preparationOrganic compound preparationNucleophileAmino radical
Owner:YUNNAN MINZU UNIV
Carbon monoxide feed gas dehydrogenation separation purification process
ActiveCN112028017ASeparation without lossReduce hydrogen contentHydrogen separation using liquid contactCarbon monoxidePtru catalystDehydrogenation
The invention relates to a carbon monoxide feed gas dehydrogenation separation purification process which comprises the following two steps: hydrogen absorption: under the action of a hydrogen absorption catalyst, absorbing hydrogen from carbon monoxide raw material gas by adopting organic liquid to obtain hydrogen absorption liquid and carbon monoxide treatment gas, wherein the organic liquid isselected from at least one of cyclohexane, tetrahydronaphthalene, carbazole and ethyl carbazole, and dehydrogenation: dehydrogenating the hydrogen absorption liquid under the action of a dehydrogenation catalyst to obtain a dehydrogenation liquid and purified hydrogen. According to the technology, organic matter is used for hydrogen absorption and hydrogen desorption, the hydrogen content in the carbon monoxide treatment gas is low and is smaller than or equal to 85 ppm, the purity of purified hydrogen is larger than or equal to 98%, and the purified hydrogen can be recycled. The method is insensitive to the concentration of hydrogen in the feed gas and can be used for treating carbon monoxide feed gas with the hydrogen volume ratio not larger than 20%, and organic liquid is recycled.
Owner:湖北浚然新材料有限公司
Method for efficiently synthesizing (1R,2S)-1,2-dialin derivative
InactiveCN108863844ALow costImprove efficiencyCarbamic acid derivatives preparationOrganic compound preparationBenzeneOrganic solvent
The invention discloses a method for efficiently synthesizing a (1R,2S)-1,2-dialin derivative. The method is characterized by carrying out asymmetric cis-form ring opening on nitrogen / oxygen benzene and norbornene by taking an oximes compound as a nucleophilic reagent in an organic solvent system and under an inertial atmosphere, thus obtaining a target object-the (1R,2S)-1,2-dialin derivative, wherein a reaction formula of the (1R,2S)-1,2-dialin derivative is as shown in the description. According to the method disclosed by the invention, efficient synthesis of the (1R,2S)-1,2-dialin-1,2-diamine derivative through one-step simple reaction by taking the simple oximes compound, the nitrogen / oxygen benzene and norbornene compounds as raw materials is realized for the first time, wherein a hydroxyl group (-OH) or amino group (-NH2) is used as a substituent group.
Owner:YUNNAN MINZU UNIV
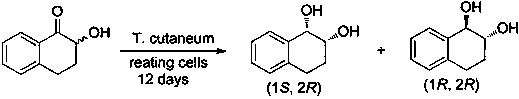
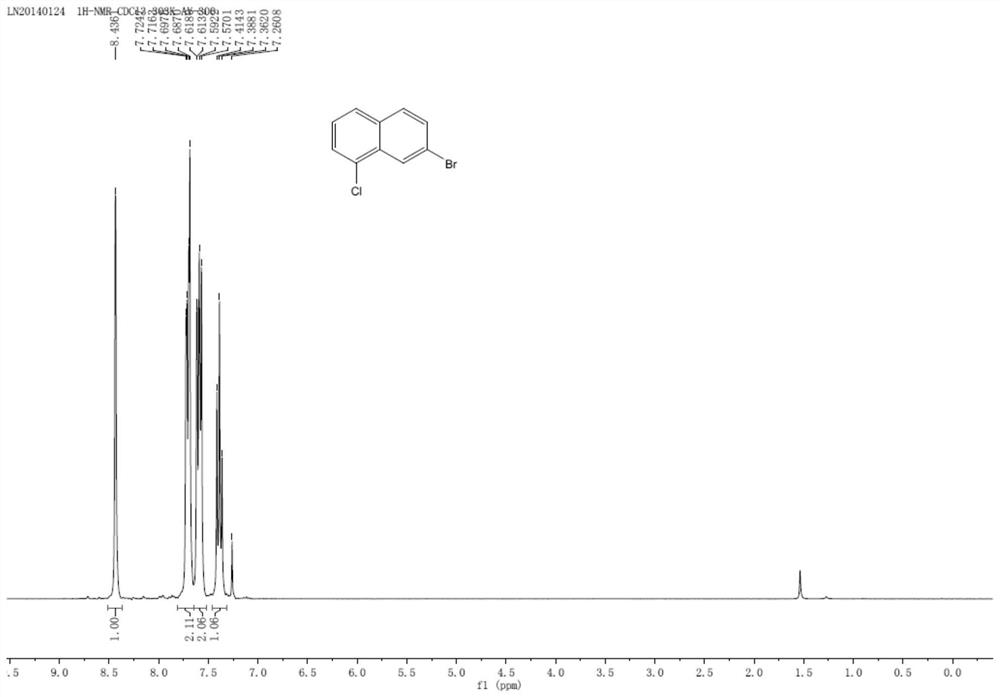
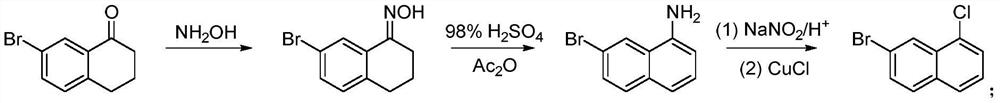
A kind of preparation method of 7-bromo-1-chloronaphthalene
ActiveCN112979412BShort reaction pathEasy to handleHalogenated hydrocarbon preparationBiochemical engineeringAromatization
The invention discloses a preparation method of 7-bromo-1-chloronaphthalene, which uses 7-bromo-3,4-dihydronaphthalene-1(2H)-one as a raw material to make 7-bromo-3,4-dihydronaphthalene Hydronaphthalene-1(2H)-ketone, phosphorus reagent and carbon tetrachloride mix, carry out chlorination reaction, generate 6-bromo-4-chloro-1,2-dihydronaphthalene; make 6-bromo-4-chloro- 1,2-dihydronaphthalene is subjected to an aromatization reaction under the action of an oxidant to generate 7-bromo-1-chloronaphthalene; this reaction route is not only shorter than the synthesis method in the prior art, but also uses safer reagents , easy to handle, easy to carry out large-scale production, good yield.
Owner:江苏丽源医药有限公司
Method for synthesizing high-density aviation kerosene from biomass diene and p-benzoquinone in one pot
PendingCN113862034AReduce dependenceHigh densityTreatment with hydrotreatment processesAlkaneCellulose
The invention relates to a method for one-pot synthesis of high-density alkyl decahydronaphthalene aviation kerosene from biomass conjugated diene and p-benzoquinone. The process is completed in one pot through two steps, in the first step, conjugated diene and p-benzoquinone are subjected to a Diels-Alder reaction under the thermal action to generate a ring adduct, and in the second step, alkyl decahydronaphthalene is synthesized through hydrodeoxygenation under the action of a bifunctional catalyst. According to the method, the separation and purification steps of an intermediate product are omitted in a one-pot manner, so that the purposes of reducing product loss, energy cost and waste output are achieved, and direct conversion from a lignocellulose platform compound to a high-density aviation kerosene alkyl decalin alkane compound is realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com