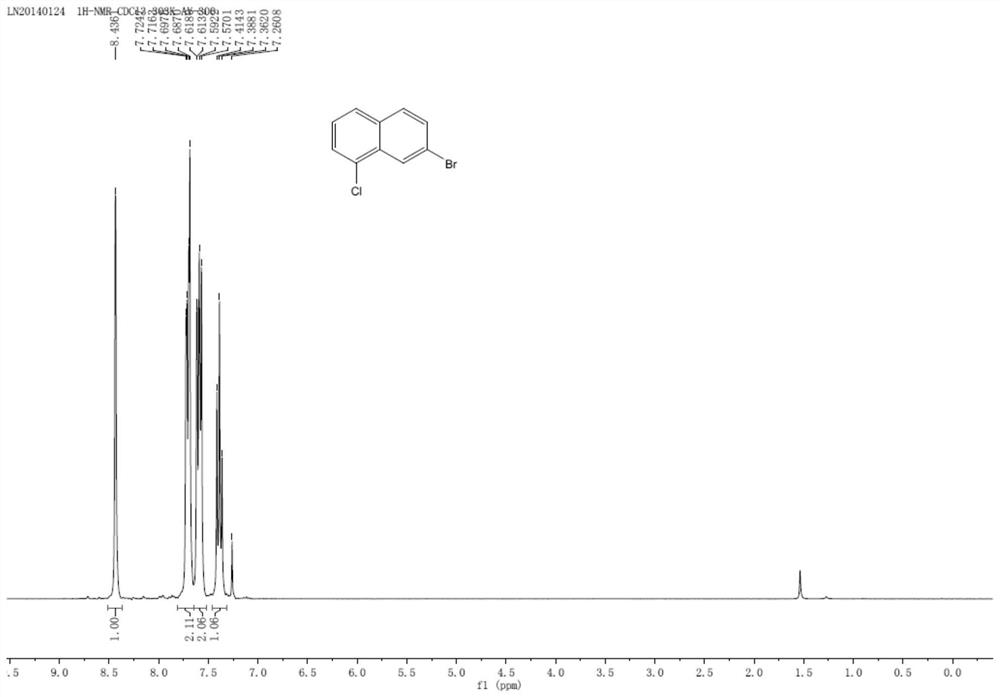
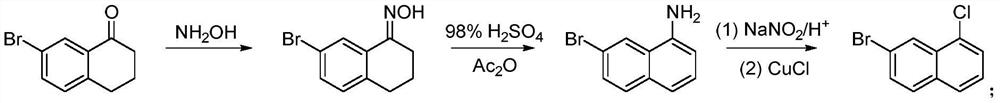
A kind of preparation method of 7-bromo-1-chloronaphthalene
A technology of chloronaphthalene and dihydronaphthalene, which is applied in the field of preparation of 7-bromo-1-chloronaphthalene, can solve the problems of long steps and high risk, and achieve the effects of short steps, easy handling and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Chlorination
[0034] Under a nitrogen atmosphere, 10 mmol of 7-bromo-3,4-dihydronaphthalene-1(2H)-one (2.25 g), 20 mmol of triphenylphosphine PPh 3 (5.24g) added to 200mmol CCl 4 (19.3 mL), stirred at room temperature for 30 min, then heated to reflux and continued the reaction for 5 h. After the reaction, it was lowered to room temperature, and CCl was added. 4 Dilute with an equal amount of n-hexane, collect the precipitated crystals by filtration, and wash with n-hexane to obtain 6-bromo-4-chloro-1,2-dihydronaphthalene (75% yield, 1.83 g, 95% purity).
[0035] Aromatization
[0036] In a nitrogen atmosphere, 5mmol of 6-bromo-4-chloro-1,2-dihydronaphthalene (1.22g) and 15mmol of DDQ (3.41g) were added to 100mmol of toluene (10.6mL) and reacted at room temperature for 16h. After the reaction, the solid was removed by filtration, the obtained solid was rotary evaporated, and recrystallized from petroleum ether to obtain the final product 7-bromo-1-chloronaphthalene...
Embodiment 2
[0038] Chlorination
[0039] 10 mmol of 7-bromo-3,4-dihydronaphthalene-1(2H)-one (2.25 g), 20 mmol of triphenoxyphosphorus (6.2 g) were added to 200 mmol of CCl under nitrogen atmosphere 4 (19.3 mL), stirred at room temperature for 30 min, then heated to reflux and continued the reaction for 5 h. After the reaction, it was lowered to room temperature, and CCl was added. 4 Dilute with an equal amount of n-hexane, collect the precipitated crystals by filtration, and wash with n-hexane to obtain 6-bromo-4-chloro-1,2-dihydronaphthalene (yield 64%, 1.56 g, purity 90%).
[0040] Aromatization
[0041] In a nitrogen atmosphere, 5mmol of 6-bromo-4-chloro-1,2-dihydronaphthalene (1.22g) and 15mmol of AIBN (2.46g) were added to 100mmol of toluene (10.6mL) and reacted at room temperature for 16h. After the reaction was completed, the solid was removed by filtration. The solid obtained by rotary evaporation of the filtrate was recrystallized from petroleum ether to obtain the final pro...
Embodiment 3
[0043] The chlorination reaction is the same as in Example 1.
[0044] Aromatization
[0045] In a nitrogen atmosphere, 5mmol of 6-bromo-4-chloro-1,2-dihydronaphthalene (1.22g) and 15mmol of TEMPO (2.34g) were added to 100mmol of toluene (10.6mL) and reacted at room temperature for 16h. After the reaction was completed, the solid was removed by filtration. The solid obtained by rotary evaporation of the filtrate was recrystallized from petroleum ether to obtain the final product 7-bromo-1-chloronaphthalene (yield 68%, 0.82 g, purity 89%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com