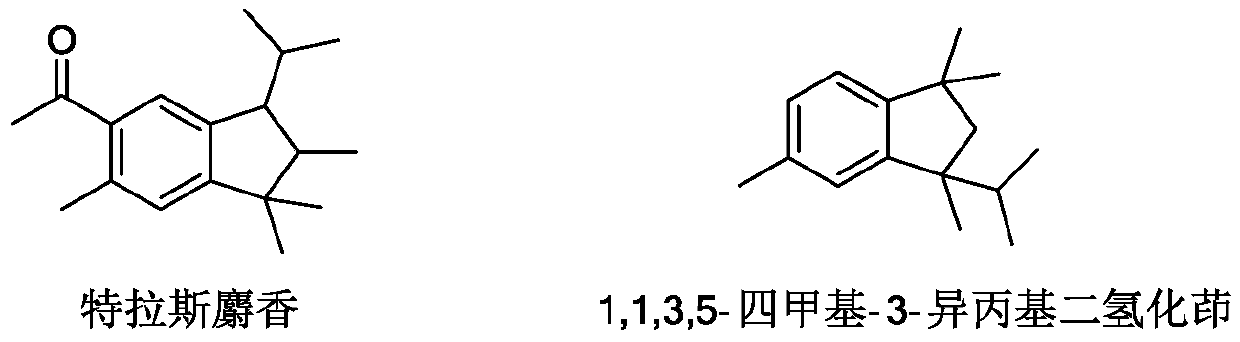
Synthesis method of 1, 1, 3, 5-tetramethyl-3-isopropyl hydrindene
A technology of isopropylindane and its synthesis method, which is applied in the fields of chemical instruments and methods, isomerization of hydrocarbons, and carbon compound catalysts, and can solve the problems of low synthesis yield of indane compounds and high difficulty in purification of target products, etc. Achieve the effect of reducing the difficulty of purification, reducing the difficulty of obtaining and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
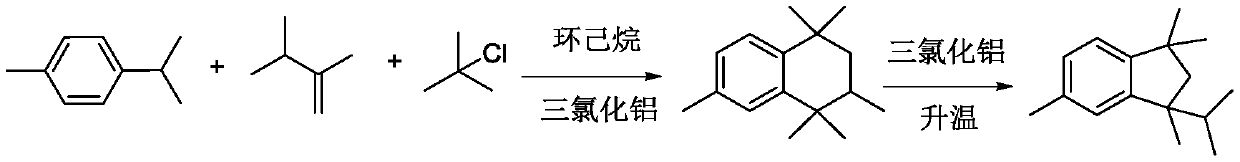
[0023] A kind of synthetic method of 1,1,3,5-tetramethyl-3-isopropylindane, specifically comprises the following steps:
[0024] S1: Mix 120g of p-cymene, 100g of tert-butyl chloride and 100g of 2,3-dimethyl-1-butene, and then add the mixture dropwise to a tank containing 350g of cyclohexane and 15g of aluminum trichloride. In a reaction flask, stir the reaction, wash with water and alkali to elute the solvent, and rectify and remove p-cymene at 100°C under a pressure of -0.09MPa. , so a large amount of cymene is removed to obtain the crude product of 1,1,3,4,4,6-hexamethyltetrahydronaphthalene;
[0025] S2: Isomerize the crude 1,1,3,4,4,6-hexamethyltetralin, then add 150g of dichloroethane and 35g of aluminum trichloride, stir and heat up to 35°C, and reflux for 1.5 hours ;
[0026] S3: The product after the isomerization reaction is washed with water and alkali to neutrality, and dichloroethane is removed by precipitation, and the 1,1,3,5-tetramethyl-3-isopropylindane afte...
Embodiment 2
[0028] S1: Mix 320g of p-cymene, 165g of tert-butyl chloride and 100g of 2,3-dimethyl-1-butene, and then add the mixture dropwise to a tank containing 400g of cyclohexane and 20g of aluminum trichloride. In a reaction flask, stir the reaction, wash with water and alkali to elute the solvent, and rectify and remove p-cymene at 120°C under a pressure of -0.095MPa to obtain 1,1,3,4,4,6-hexamethyltetra crude hydrogenated naphthalene;
[0029] S2: Isomerize the crude 1,1,3,4,4,6-hexamethyltetralin, then add 240g of dichloroethane and 28g of aluminum trichloride, stir and raise the temperature to 55°C, and reflux for 2 hours ;
[0030] S3: The product after the isomerization reaction is washed with water and alkali to neutrality, and dichloroethane is removed by precipitation, and the 1,1,3,5-tetramethyl-3-isopropylindane after precipitation is Recrystallize the crude product, add 80g of crystallization solvent, raise the temperature to 50°C, lower the temperature to 30°C for 2h a...
Embodiment 3
[0032] S1: Mix 500g of p-cymene, 500g of tert-butyl chloride and 100g of 2,3-dimethyl-1-butene evenly, then add the mixture dropwise to a tank containing 700g of cyclohexane and 25g of aluminum trichloride In a reaction flask, stir the reaction, wash with water and alkali to elute the solvent, and rectify and remove p-cymene at 120°C under a pressure of -0.1MPa to obtain 1,1,3,4,4,6-hexamethyltetra crude hydrogenated naphthalene;
[0033] S2: Isomerize the crude 1,1,3,4,4,6-hexamethyltetralin, then add 300g dichloroethane and 90g aluminum trichloride, stir and heat up to 55°C, and reflux for 2 hours ;
[0034] S3: The product after the isomerization reaction is washed with water and alkali to neutrality, and dichloroethane is removed by precipitation, and the 1,1,3,5-tetramethyl-3-isopropylindane after precipitation is Recrystallize the crude product, add 100g crystallization solvent, raise the temperature to 50°C, lower the temperature to 30°C for 2h at a stirring rate of 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com