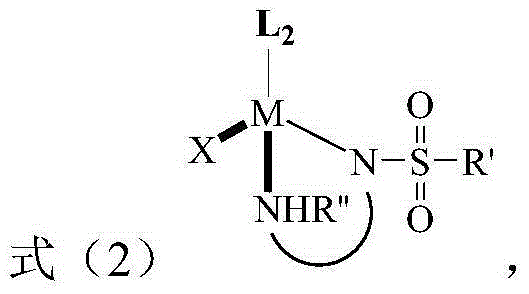Preparation method of tetrahydro 1, 8-naphthyridine compound and chiral product prepared by adopting preparation method
A compound and naphthyridine technology are applied in the preparation of tetrahydro 1,8-naphthyridine compounds and the field of the prepared chiral products, which can solve problems such as catalyst poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
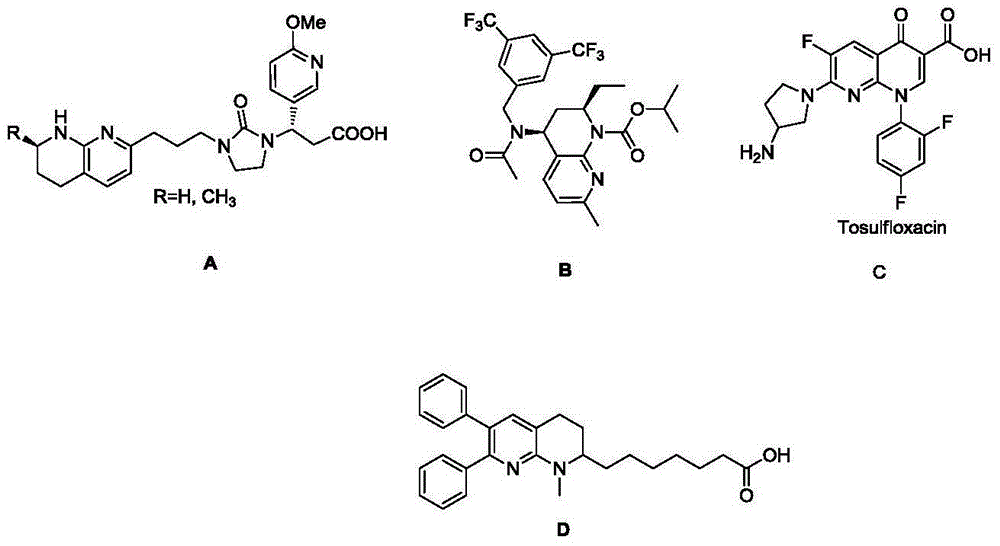
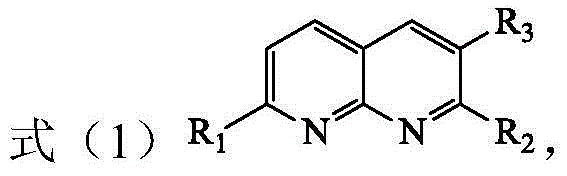
[0021] The present invention provides a method for preparing tetrahydro-1,8-naphthyridine compounds, wherein the method comprises: in the presence of a chiral catalyst, performing an addition reaction of a compound represented by formula (1) with hydrogen, wherein , the chiral catalyst is a complex of the structure shown in formula (2);
[0022]
[0023] Among them, R 1 , R 2 and R 3 each independently hydrogen, substituted or unsubstituted C1-C10 alkyl, substituted or unsubstituted C3-C10 cycloalkyl, substituted or unsubstituted aryl or substituted or unsubstituted arylbenzyl base, or R 2 and R 3 Connect to form a C5-C8 membered alkane ring, wherein, the substituents in the substituted alkyl, substituted cycloalkyl, substituted aryl and substituted arylbenzyl are each independently selected from fluorine, chlorine, bromine, nitro One or more of radical, methyl, methoxy, trifluoromethyl, hydroxyl and acetamido; and, R 1 not hydrogen;
[0024]
[0025] Wherein, M i...
preparation example 1
[0137] (1) Dissolve (R,R)-1,2-diphenyl-ethylenediamine (20mmol, purchased from Bailingwei Technology Co., Ltd. 24694 brand) in dichloromethane (30mL), and dissolve in P-methylphenylsulfonyl chloride (20mmol, purchased from Bailingwei Technology Co., Ltd. 283322 brand) in dichloroethane (30mL) was added dropwise (dropped within 30min), continued to react at 0°C for 1h, and then reduced pressure Rotary evaporation, the solid was separated and purified by column chromatography (the eluent was dichloromethane / methanol with a volume ratio of 10:1), so as to obtain 15 mmol of the formula (R, R)-(3-1-1-1) The chiral diamine shown has a yield of 75%, and the identification data of this chiral diamine is: 1 HNMR (300MHz, CDCl 3 ): δ7.31(d, J=8.3Hz, 2H), 7.18-7.09(m, 10H), 6.97(d, J=8.3Hz, 2H), 4.37(d, J=5.2Hz, 1H), 4.12 (d,J=5.2Hz,1H),2.32(s,3H),1.49(br,3H); 13 CNMR (75MHz, CDCl 3 ): δ142.5, 139.2, 137.2, 129.1, 128.4, 128.2, 127.5, 127.4, 127.0, 126.9, 126.6, 63.2, 60.5, 21.4.
...
preparation example 2
[0141] (1) According to the method of step (1) in Preparation Example 1, the difference is that methanesulfonyl chloride (20mmol, purchased from Alfa Aisha Chemical Co., Ltd. A13383 brand) is used instead of p-methylphenylsulfonyl chloride, and Reaction at 0°C for 1 h, thereby obtaining 14.6 mmol of chiral diamine represented by the formula (R,R)-(3-1-1-2), with a yield of 73%. The identification data of the chiral diamine for: 1 HNMR (300MHz, CDCl 3 ): δ7.34-7.26(m, 10H), 4.56(d, J=5.1Hz, 2H), 4.21(d, J=5.1Hz, 2H), 2.26(s, 3H); 13 CNMR (75MHz, CDCl 3 ): δ141.9, 139.7, 128.7, 128.6, 127.9, 127.8, 126.9, 126.7, 63.4, 60.2, 40.7.
[0142] (2) According to the method of step (2) in Preparation Example 1, the difference is that the chiral diamine used is the chiral diamine shown in the above formula (R, R)-(3-1-1-2) (145 mg, 0.5 mmol), recrystallized to obtain 275 mg of a red solid (ie, the complex shown in formula (R, R)-4j);
[0143] (3) According to the method of step (3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com