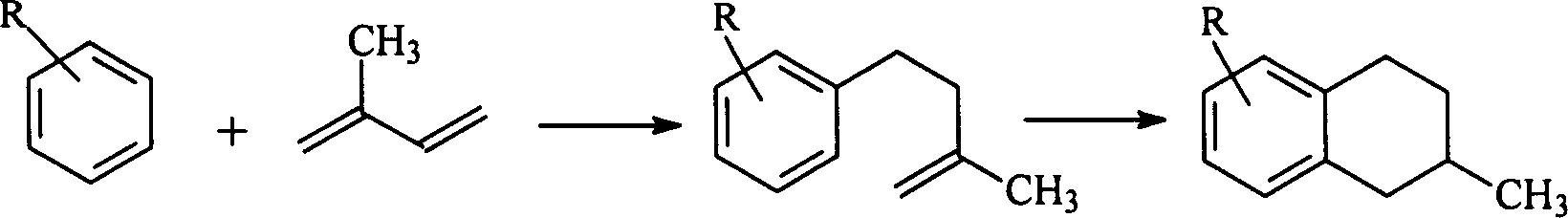
Method of synthesizing alkyl methyl tetra hydronaphthalene using alkyl benzene and isopentadiene as raw material
A technology of alkyl methyl tetralin and isoprene, applied in the field of alkyl methyl tetralin, can solve the problems of high price, increase the complexity of the reaction and the like, and achieve the effect of simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0018] In a batch reactor with a volume of 5 liters, with stirring device and heating device, add 1.5L alkylbenzene and the catalyst of required amount, control the pressure of the reactor, and the material in the reactor is heated to the required temperature reflex. After starting the stirring, slowly add the required amount of isoprene into the reactor to carry out the alkylation reaction. The reaction start time was counted from the addition of isoprene, and the reaction was terminated after the required reaction time.
[0019] After the reaction product is discharged, the catalyst is separated (if the catalyst adopts Y-type molecular sieve, the separation of the catalyst is not carried out), then the reaction product is placed in the above-mentioned reactor, and the required amount of catalyst Y-type molecular sieve is added to carry out the ring-forming reaction, and the reaction is controlled. The pressure of the device and the heating temperature of the material. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com