Method for forming metal oxide coating film and vapor deposition apparatus
a technology of metal oxide coating film and vapor deposition apparatus, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, coatings, etc., can solve the problem of inability to control the hydrolysis reaction in the gas phase, and achieve the improvement of the utilization factor of ticl4, uniform thickness, and good appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
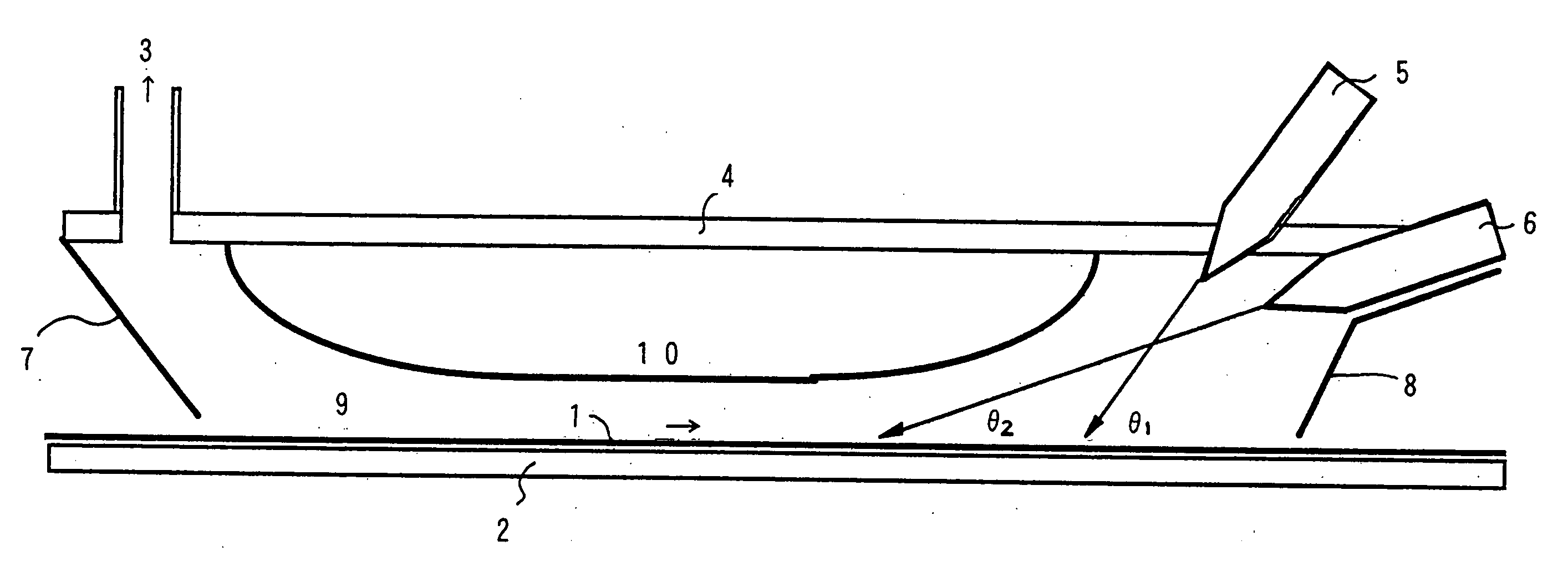
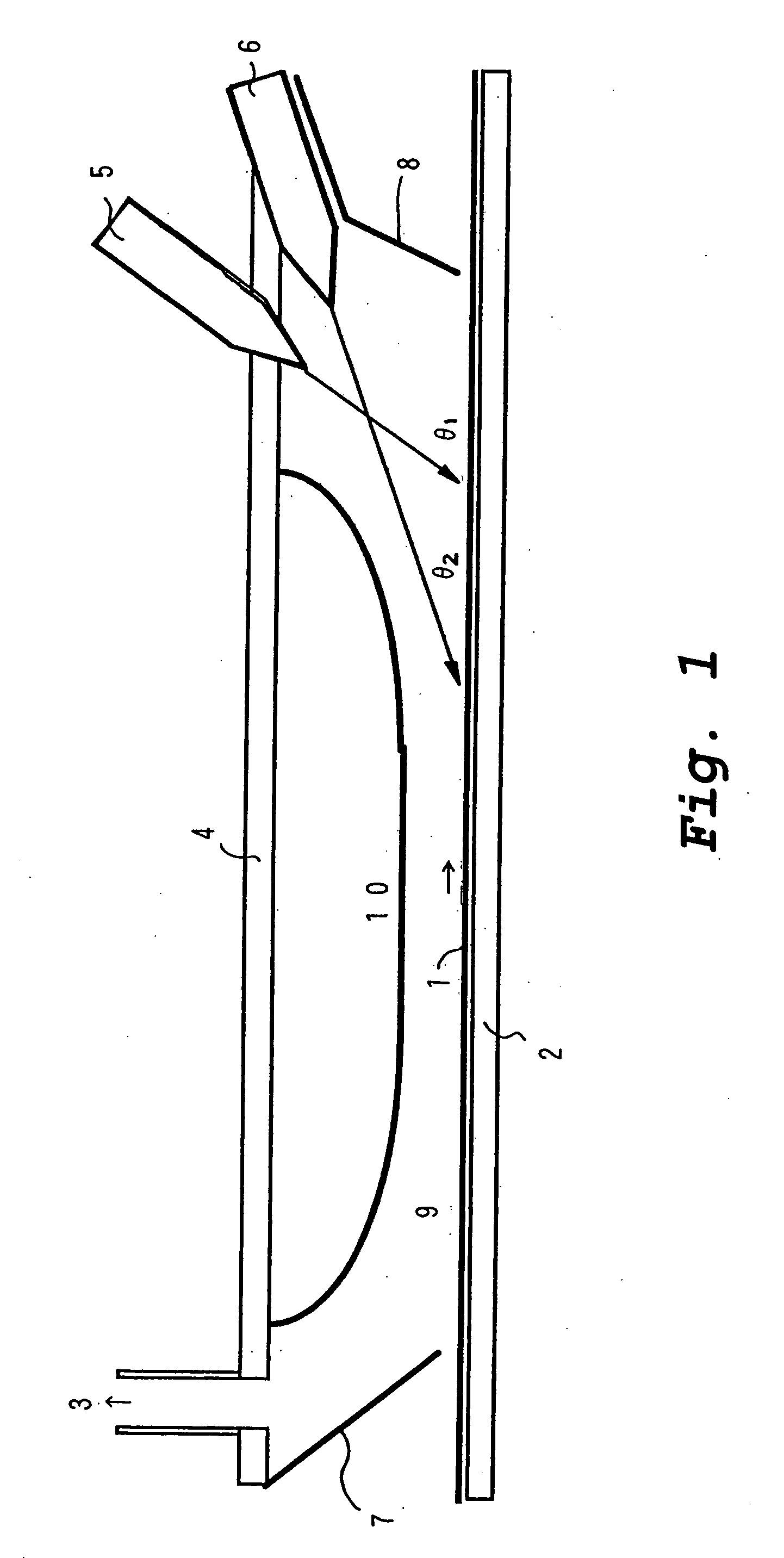
[0055] A quartz plate used as a substrate was placed at an angle of 20° with respect to the horizontal in a quartz tube which was disposed horizontally. At one end of the quartz tube, TiCl4 vapor and water vapor used as reactants for vapor deposition were injected through respective single-orifice nozzles to form jetted streams of vapor and were discharged from the other end of the tube to perform a vapor deposition test.
[0056] The TiCl4 vapor and the water vapor were diluted with argon and dry air, respectively, to form each into a diluted vapor having a concentration of 3%, which was used for injection. The atmosphere in the tube before injection of the vapors was an argon atmosphere. The temperature of the substrate was made 180° C. by heating the tube at 180° C. with an external heater. In order to prevent condensation of the TiCl4 vapor and the water vapor, each diluted vapor was heated by heating its feed pipe to the inlet of the quartz tube at 60° C. The two nozzles were til...
example 2
[0061] Vapor deposition and calcination were carried out in accordance with the procedure in Example 1 except for the following points. In this example, the position of the substrate in the quartz tube was fixed such that the time elapsed from mixing of the TiCl4 vapor and water vapor until contact of the mixed vapors with the substrate was 2 seconds, while the temperature of the substrate was varied in the range of from 100-500° C. The temperature of the substrate was controlled at a predetermined temperature by heating the quartz tube from the outside in an electric oven. For the same purpose as described in Example 1, the feed pipe of each diluted vapor to the inlet of the quartz tube was heated at 60° C. or higher.
[0062] The photocatalytic activity of each titanium oxide film formed on the substrate was evaluated by the aldehyde decomposition test described below. In addition, the adhesion of the titanium oxide film was tested by a tape peel test and evaluated as follows: mark ...
example 3
[0065] Vapor deposition and calcination were carried out in the same manner as in Example 2 except for the following points. In this example, the temperature of the substrate was 180° C., which was the same as in Example 1, but the ratio of TiCl4 vapor to water vapor injected into the quartz tube (H2O / TiCl4 molar ratio) was varied.
[0066] The rate of film formation and the photocatalytic activity of each titanium oxide film formed on the substrate were evaluated as described in Examples 1 and 2, respectively, and the results are shown in Table 3.
TABLE 3Rate of aldehydeRate of filmH2O / TiCl4decompositionformationmolar ratio[ppm / min][nm / min]0.10.79500.51.9520011.561502113030.8511050.7975100.8350
[0067] As can be seen from Table 3, at a H2O / TiCl4 molar ratio of 5 or greater, the rate of film formation decreased. In view of the rate of film formation and the photocatalytic activity, the molar ratio is preferably 3 or lower, and in particular, at a molar ratio of about 0.5, both the rate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com