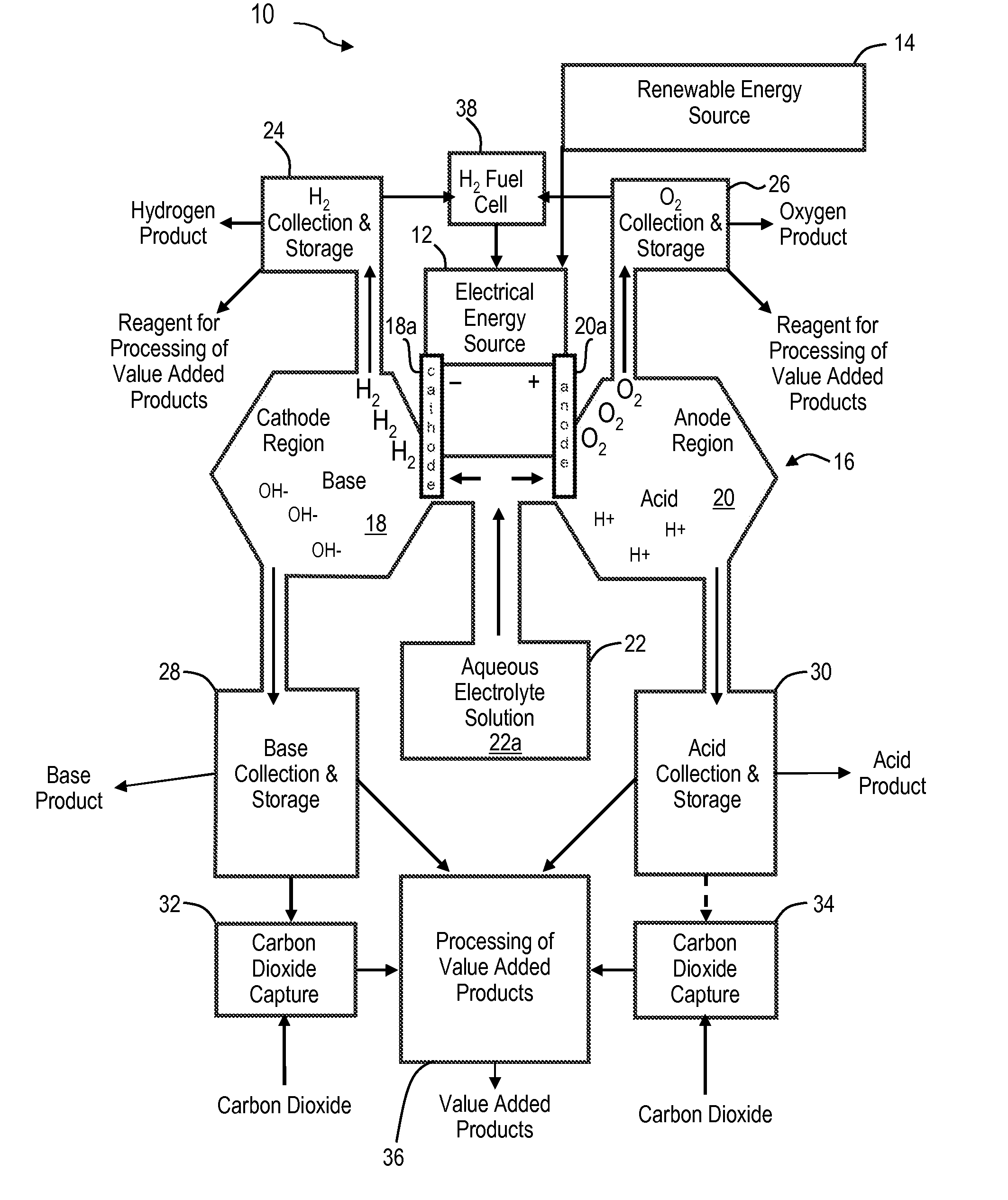
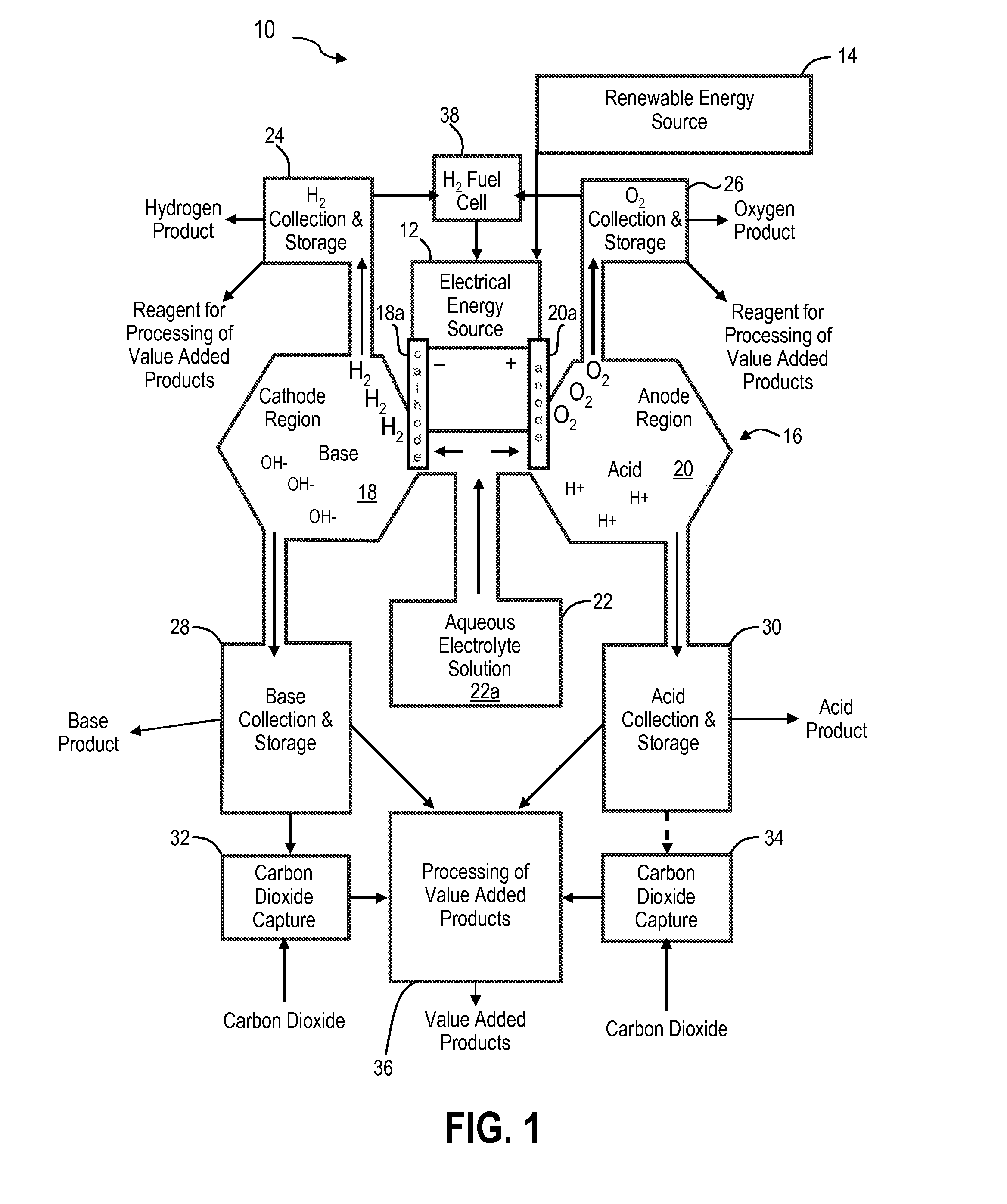
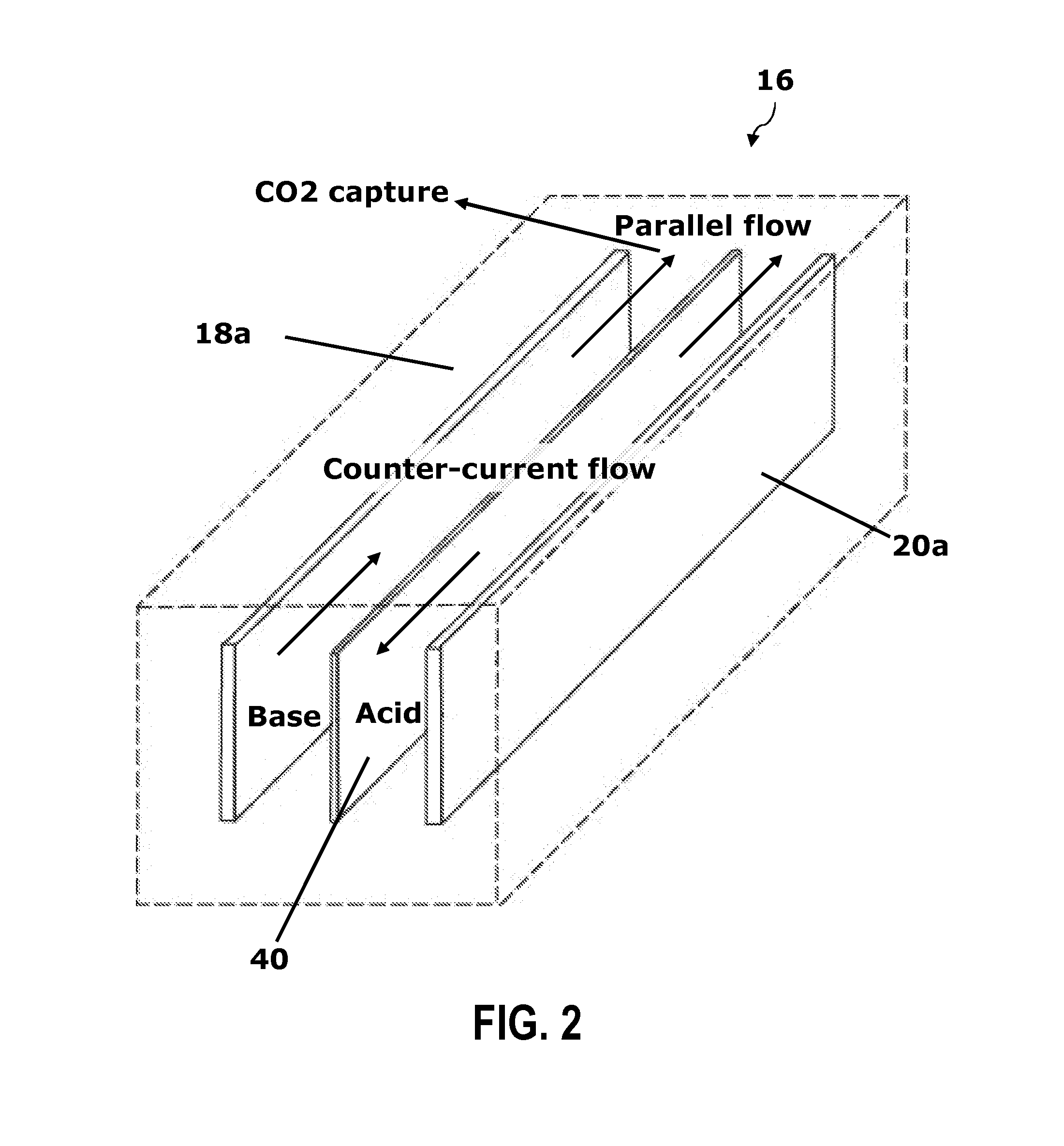
Renewable energy system for hydrogen production and carbon dioxide capture
a technology of carbon dioxide capture and hydrogen production, applied in the direction of energy input, waste based fuel, electrochemical generators, etc., can solve the problems of generating several tons of carbon dioxide pollution per ton of manufactured base, abundant chlorine, toxic, etc., and achieve the effect of eliminating atmospheric carbon and no carbon dioxid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0044]A water electrolysis cell, shown in FIG. 6, was constructed to demonstrate the feasibility of generating concentrated acid and base for carbon dioxide trapping. It consisted of a vertical central electrolyte feed tube about 2.5 centimeters (cm) in diameter, connected near its base to upward slanting anode and cathode tubes attached opposite one another. Wire, screen or flat, linear electrodes consisting of nickel, stainless steel or platinum were placed in the anode and cathode tubes near their points of attachment to the central tube. A concentrated, chloride-free electrolyte solution of aqueous sodium sulfate was introduced to the system via the central feed tube, creating an electrically conductive cell in which water was oxidized at the anode and reduced at the cathode. A small 15-watt solar panel was used to provide renewable electricity to the system.
[0045]When a DC current from the solar panel was applied to the system, hydrogen and hydroxide base were produced rapidly ...
example 2
[0048]A second example used a 1-inch diameter glass tube sealed at the bottom with a porous glass frit. The frit allowed fluid and ion exchange between the inside and outside of the glass tube, creating an inner anode or cathode cell. Flat nickel or platinum electrodes were placed on opposite sides of the glass frit and attached to a 15 W DC photovoltaic panel or DC power supply. This system created a water electrolysis device that produced concentrated base inside the tube and concentrated acid outside the tube.
[0049]Depending on mode of operation, a pH differential of over 11 was quickly generated in this system; an acid-base concentration gradient of over 20 billion fold. The electrolyte inside the tube reached a pH of about 13, while across the frit, less than ¼ inch away, the electrolyte pH reached about 1.6. Vigorous production of hydrogen and oxygen were also observed.
example 3
[0050]A third example included a two-chamber flow-through system constructed from machined plastic. A peristaltic pump was used to circulate electrolyte solution into the anode and cathode chambers, which were physically separated by a semi-permeable membrane or filter. Variable width plastic spacers were used to vary the gaps between the electrodes and the membrane. A nickel-copper alloy was initially used as electrode material. Hydrogen and oxygen were collected at valves at the top of the device, and acid and base were continually circulated past the electrodes until sufficient concentrations were reached. A variable output DC power source was used to generate voltages sufficient to electrolyze water.
[0051]pH differentials of over about 10 units were quickly achieved and maintained in this system. The nickel-copper electrodes proved susceptible to corrosion at certain voltages. Corrosion-resistant nickel, platinum, alloy or stainless steel electrodes would be more suitable for us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| DC voltages | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com