Integrated catalytic process for converting alkanes to alkenes and catalysts useful for same
A technology for catalysts and dehydrogenation catalysts, applied in the directions of carbon compound catalysts, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
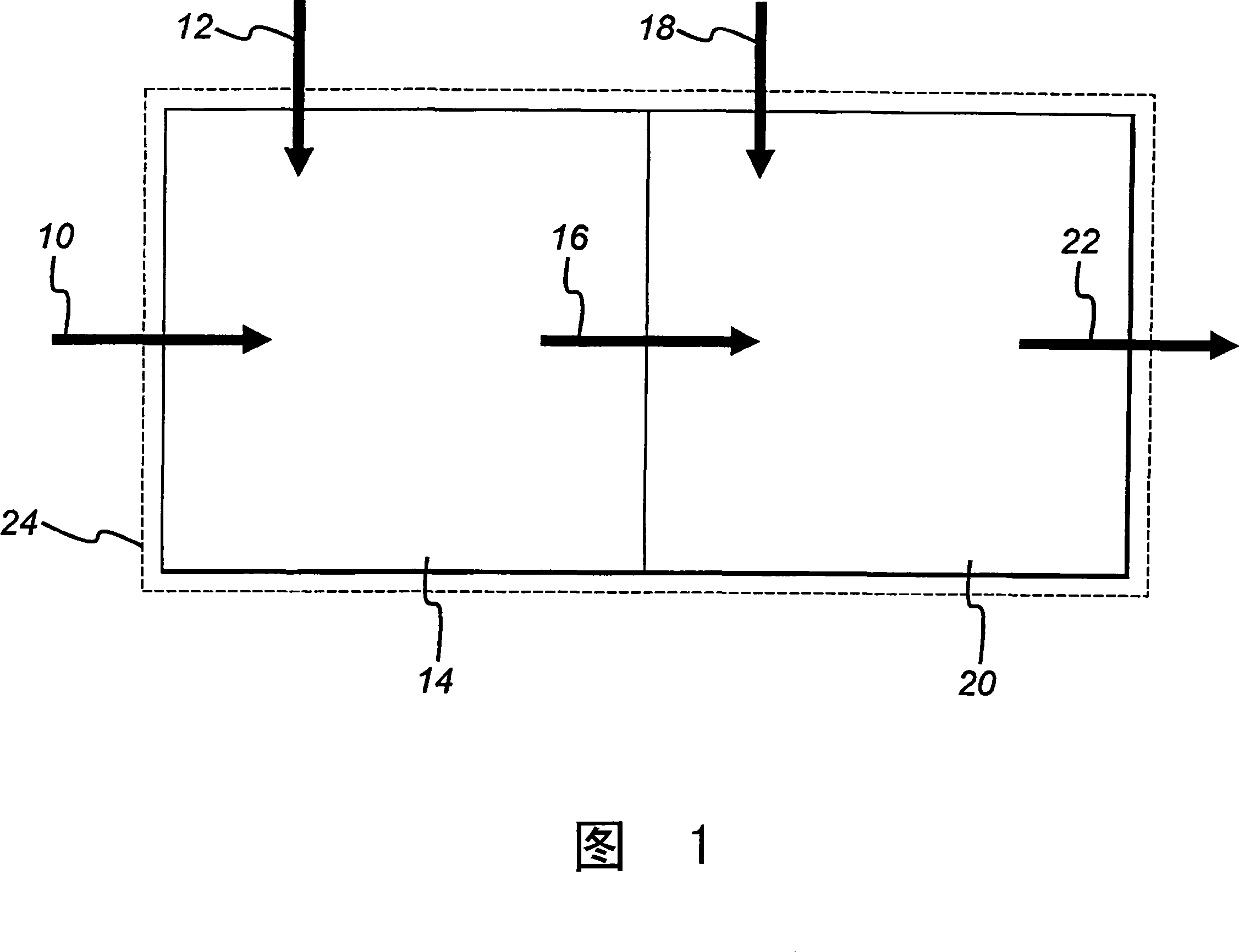
[0046] The method of the present invention includes sequential thermally combined reactions, each reaction will have a specific C 2 -C 4 Conversion of alkanes to their corresponding C 2 -C 4 Olefin. This method makes the corresponding C 2 -C 4 The total yield of olefins and the total thermal efficiency are higher than the results obtained by either reaction alone. The method and the catalyst suitable for the method will be described in general below. Then, an exemplary embodiment of the present invention will be described in more detail. The method of the present invention is a combination of an oxidative dehydrogenation reaction for converting propane to propylene and an endothermic dehydrogenation reaction. Although exemplary embodiments will be described in detail below, those of ordinary skill in the art can appreciate and understand that according to the ordinary skills and general knowledge of those skilled in the art, the present invention can be used for other types of rea...
Embodiment 1
[0082] Put 2ml (ml) of the V / Nb / Si / Ox oxidative dehydrogenation catalyst prepared by the above method in the exothermic reaction zone, and use 2ml of the V / Cr / Ag / Si / Ox endothermic dehydrogenation catalyst for the endothermic reaction Area. Each catalyst was independently tested under the conditions of 625°C and a residence time of 0.4 seconds. The catalyst V / Nb / Si / Ox in the first zone was evaluated with feed A, and the catalyst V / Cr / Ag / Si / Ox in the second zone was tested with feed B. The mixing test (ie including both catalysts at the same time) was carried out with feed A. The results of these tests are shown in Table 1. As shown in the table, the cumulative yield of the mixed method is higher than the yield of any reaction zone when operating alone.
[0083] Table 1: 625°C, residence time 0.4 seconds, using feed A
[0084] Reaction zone
Catalyst
T℃
C%
S%
Y%
ODH
V / Nb *
625
30.3
55.8
16.9
...
Embodiment 2
[0090] The second test was carried out using the same amount of the same catalyst as in Example 1, but with a residence time of 0.6 seconds. The results are listed in Table 2 below. As shown in the table, the cumulative yield of the mixed method is higher than the yield of any reaction zone when operating alone.
[0091] Table 2: 522°C, residence time 0.6 seconds, using feed A
[0092] Reaction zone
Catalyst
T℃
C%
S%
Y%
ODH
V / Nb * +
577
47.3
56.8
27.0
Endothermic
V / Cr / Ag **
582
25.9
92.7
24.0
Hybrid-both
V / Nb and / Cr / Ag
522
36.7
83.9
31.0
[0093] Temperature=T°C; Propane conversion rate=C%; Selectivity to propylene=S%; Propylene yield=Y%
[0094] * Independently with feed A
[0095] ** Independently with feed B
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com