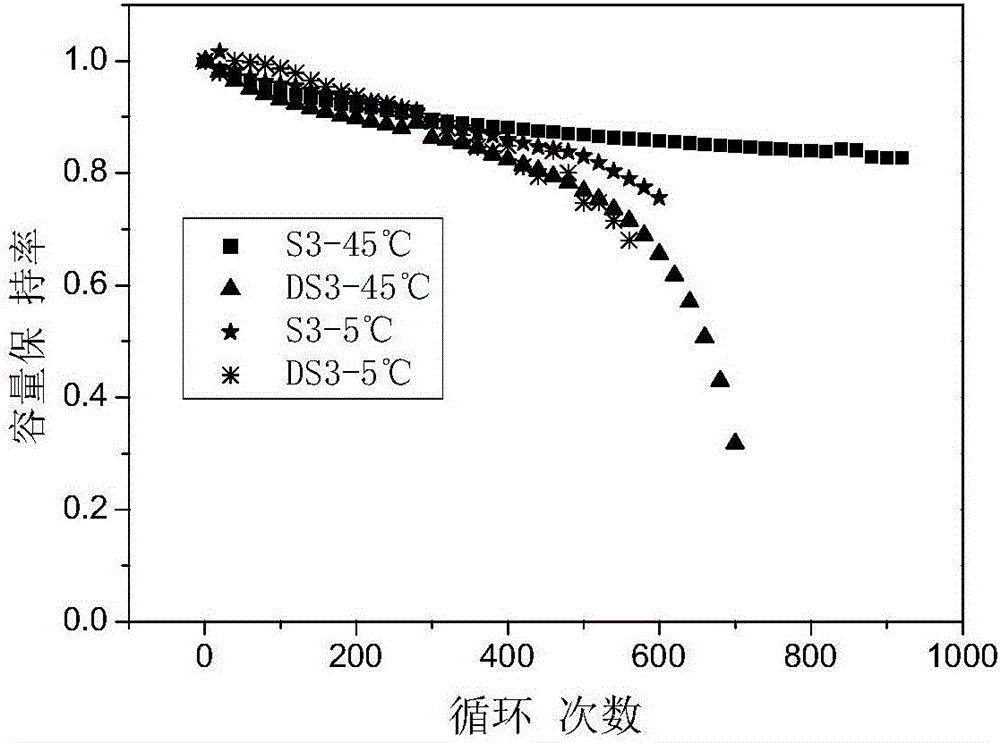
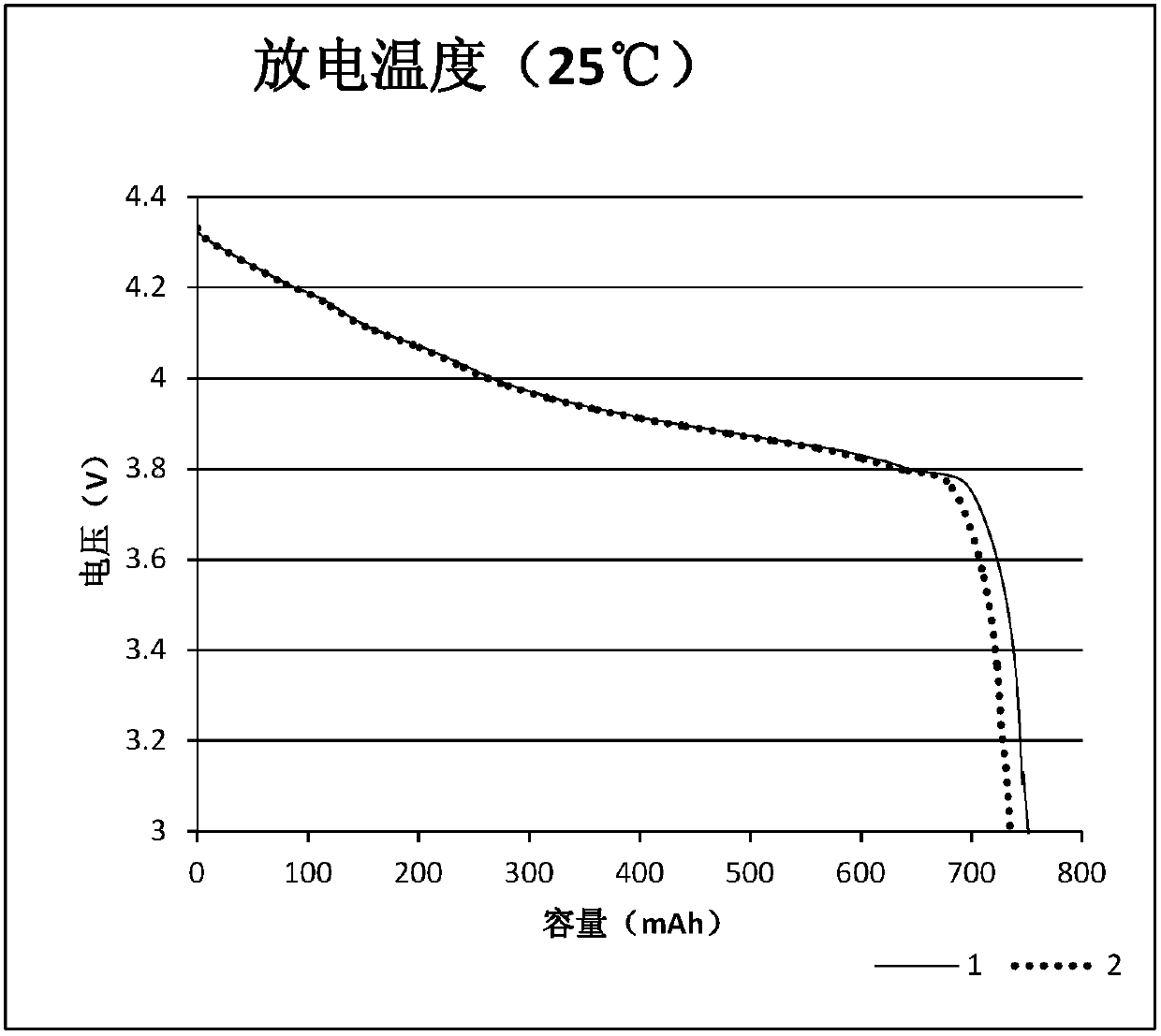
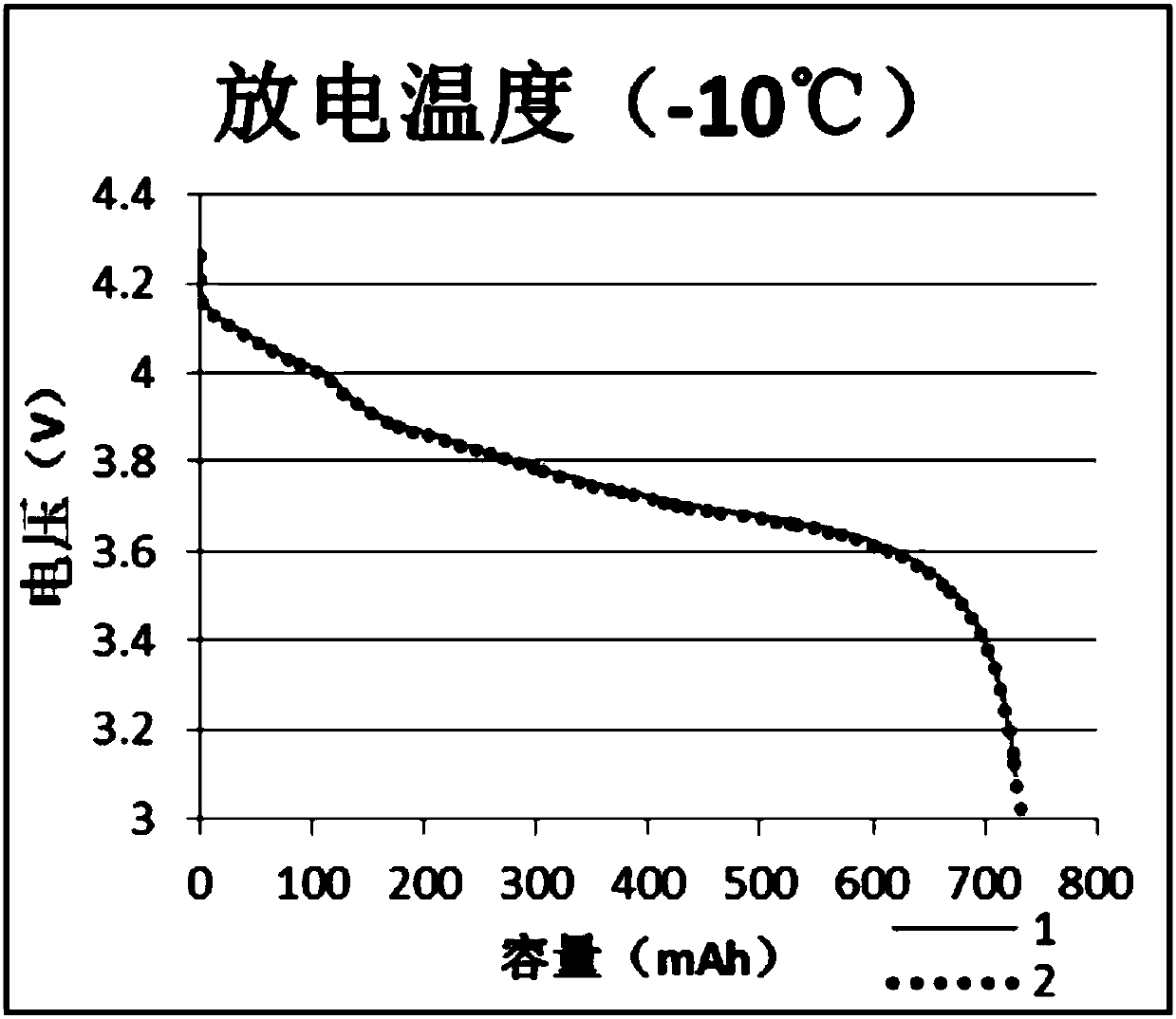
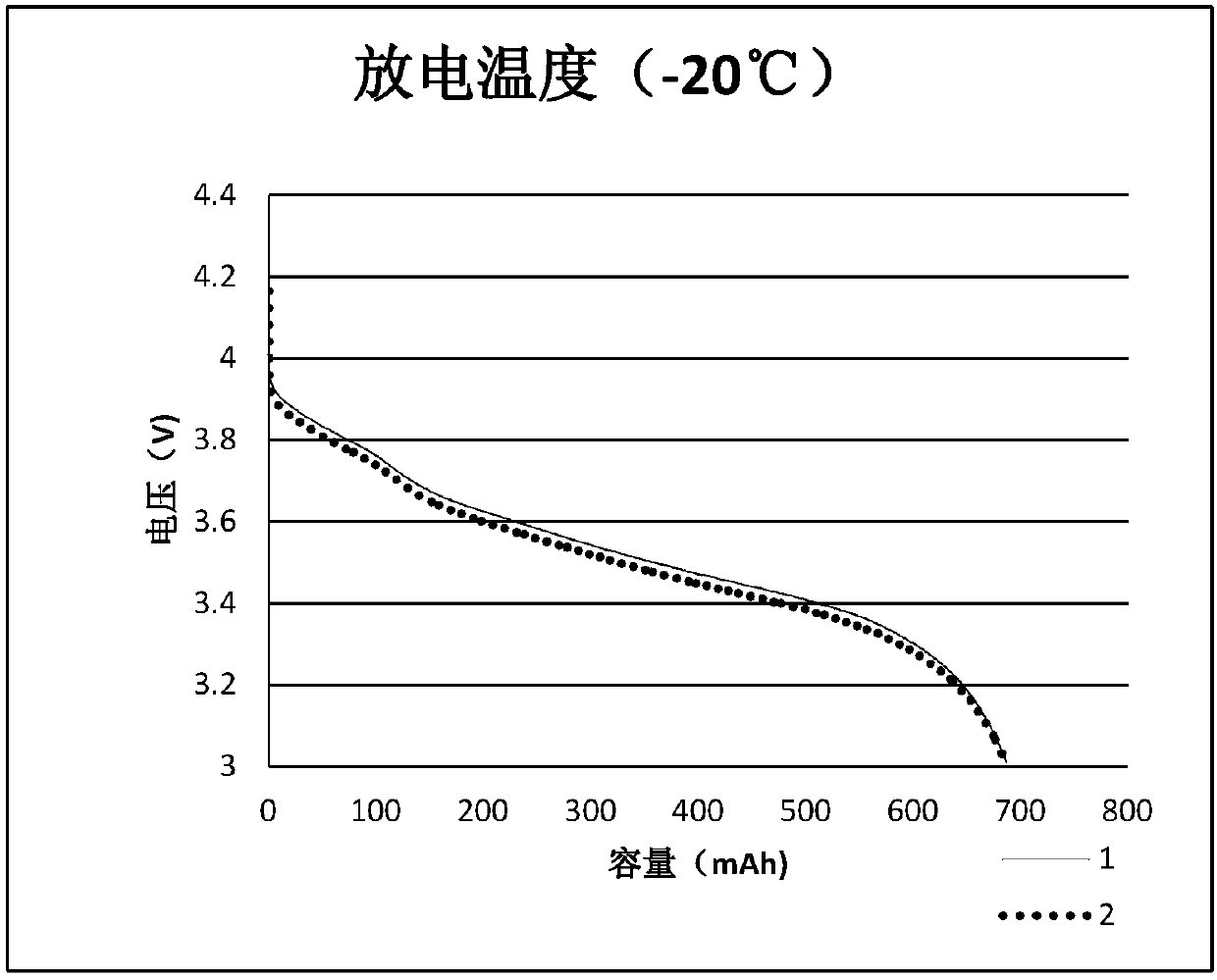
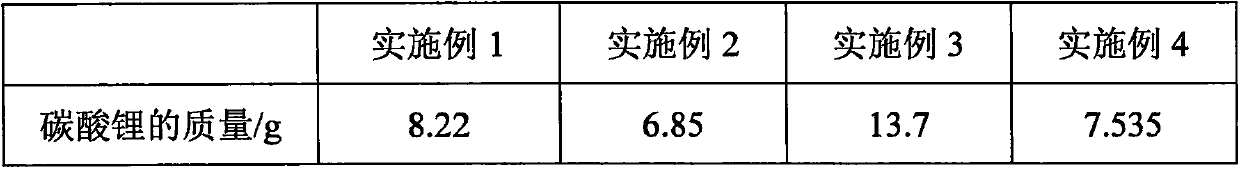
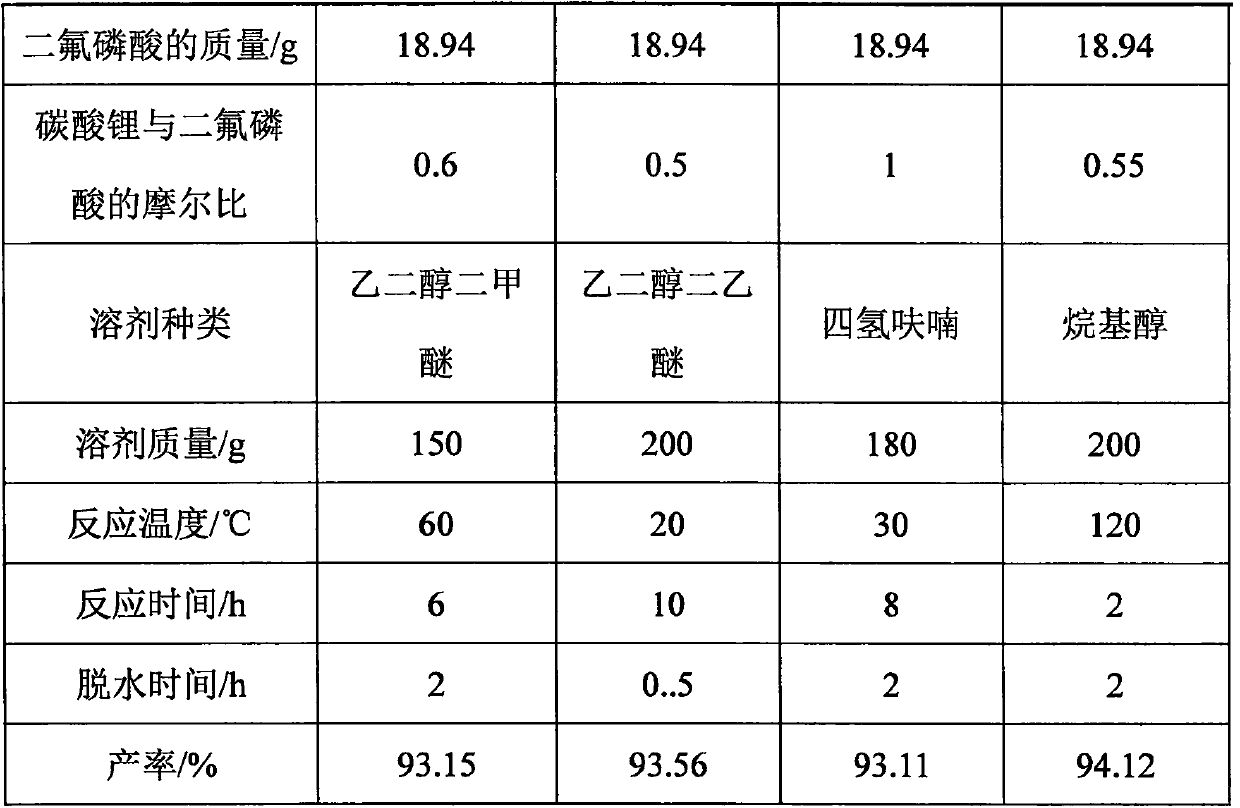
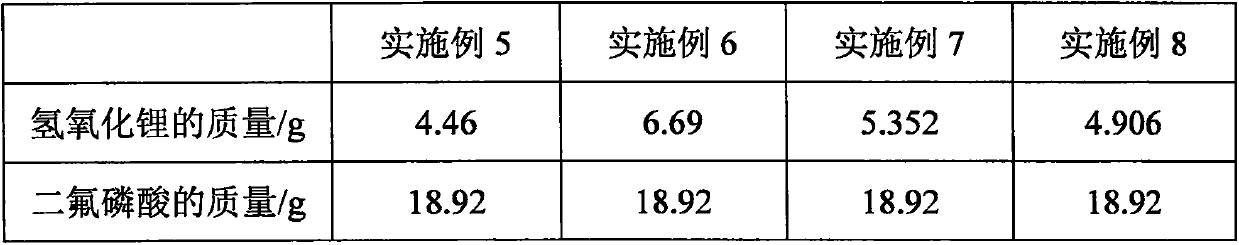
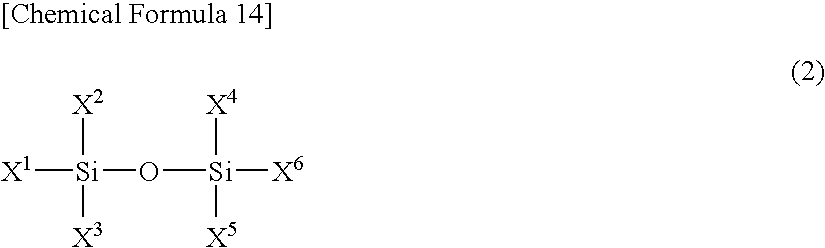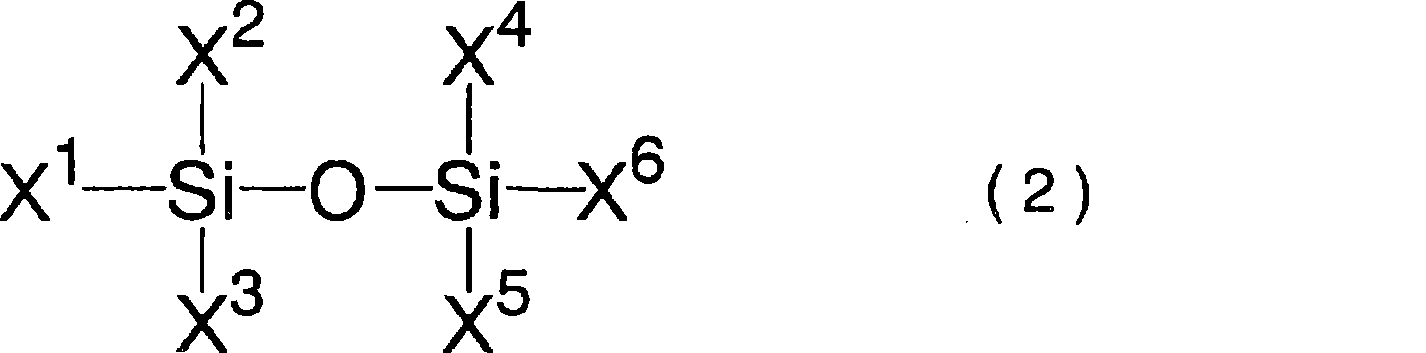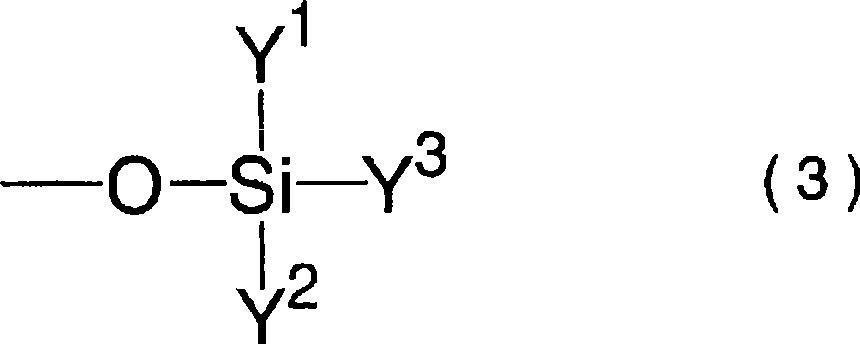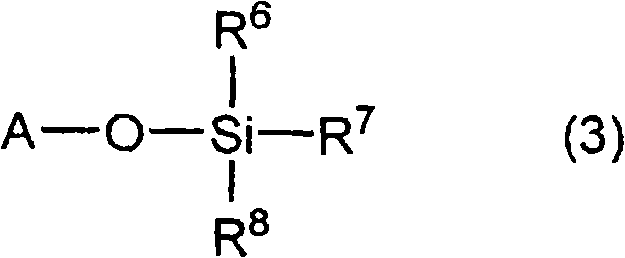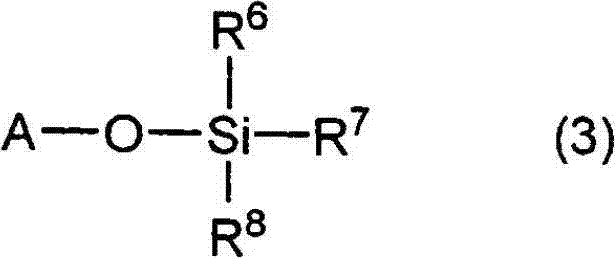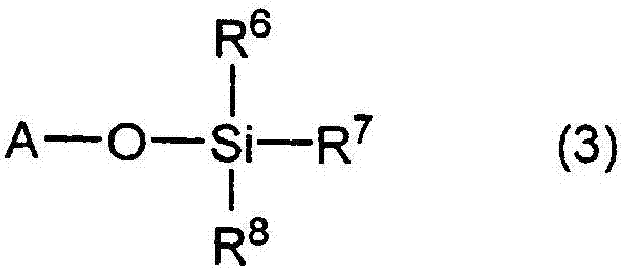Patents
Literature
257 results about "Difluorophosphoric acid" patented technology
Efficacy Topic
Property
Owner
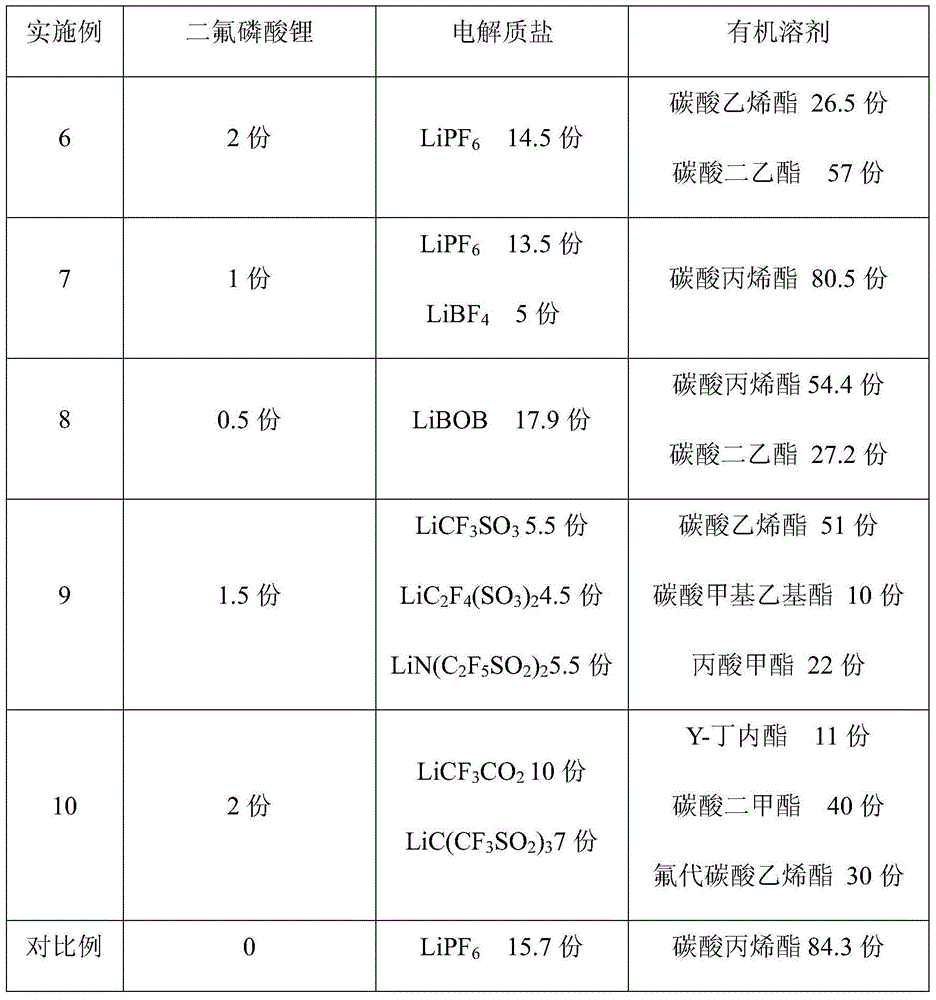
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
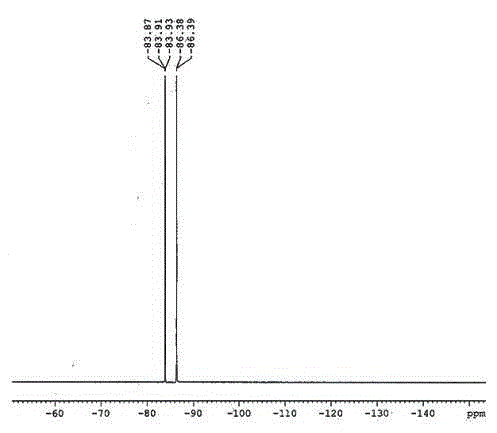
Difluorophosphoric acid is an inorganic compound with the formula HPO 2 F 2. It is a colorless liquid. It is a colorless liquid. The acid has limited applications, in part because it is thermally and hydrolytically unstable.
Lithium difluorophosphate, electrolyte containing lithium difluorophosphate, process for producing lithium difluorophosphate, process for producing nonaqueous electrolyte, nonaqueous electrolyte, and nonaqueous electrolyte secondary battery containing the same
InactiveUS20090286155A1Good low temperatureExcellent cycle characteristicsPhosphorus halides/oxyhalidesCell electrodesBoiling pointSolvent
A difluorophosphate salt, which is expensive and not readily available, can be produced with a high purity readily and efficiently from inexpensive and readily available materials. A nonaqueous electrolyte secondary battery that exhibits low-temperature discharge and heavy-current discharge characteristics and high-temperature preservation and cycle characteristics without impairing the battery safety. A hexafluorophosphate salt is reacted with a compound having a bond represented by the following formula (1) in the molecule:Si—O—Si (1)A nonaqueous electrolyte used for nonaqueous electrolyte secondary batteries including a negative electrode and a positive electrode that can occlude and discharge ions, and a nonaqueous electrolyte is prepared from a mixture obtained by mixing at least one nonaqueous solvent, a hexafluorophosphate salt and a compound having a bond represented by the following formula (1), and removing low-boiling compounds newly formed in the system, the low-boiling compounds having a lower boiling point than that of the compound having the bond represented by the formula (1):Si—O—Si (1)
Owner:MITSUBISHI CHEM CORP
Method for Producing Difluorophosphate, Nonaqueous Electrolyte Solution for Secondary Battery and Nonaqueous Electrolyte Secondary Battery
ActiveUS20080102376A1Phosphorus halides/oxyhalidesNon-aqueous electrolyte accumulatorsDifluorophosphateSource material
The present invention provides a simple method for producing a difluorophosphate from a source material, the difluorophosphate being useful as additives for nonaqueous electrolyte solutions for secondary batteries. In the method, a source material containing a carbonate and / or a borate is allowed to react with a source gas which contains P and F and which may further contain O as required. The source material may contain lithium carbonate. The source gas may be produced by decomposing LiPF6. The source gas may be produced in such a manner that LiPF6 and lithium carbonate are mixed and then subjected to reaction. The nonaqueous electrolyte solution contains the product obtained from the reaction.
Owner:MU IONIC SOLUTIONS CORP +1
High-capacity lithium-ion battery electrolyte of considering high-and-low temperature performance, preparation method and lithium-ion battery
InactiveCN106252639AImprove bindingConsider high temperature performanceCell electrodesSecondary cellsCarbon compositesHigh temperature storage
The invention discloses a high-capacity lithium-ion battery electrolyte of considering high-and-low temperature performance. The electrolyte comprises a non-aqueous solvent, lithium hexafluorophate, a negative film-forming additive, an inflatable inhibition additive and a low-impedance additive, wherein the negative film-forming additive is prepared from fluoroethylene carbonate which accounts for 3%-15% of total mass of the electrolyte; the inflatable inhibition additive is prepared from one or two of 1,3-propene sultone or anhydride compounds which account for 0.3%-5% of total mass of the electrolyte; and the low-impedance additive is prepared from one or two of lithium difluorophosphate and difluoride phosphate lithium oxalate which account for 0.2%-3% of total mass of the electrolyte. The electrolyte is suitable for a high-nickel positive electrode and silicon-carbon composite negative electrode lithium-ion battery; the high-temperature storage performance and the low-temperature discharge performance of the lithium-ion battery are improved while the room-temperature cycle performance is considered; and meanwhile, the invention further provides a preparation method of the electrolyte and the high-capacity lithium-ion battery of using the electrolyte.
Owner:GUANGZHOU TINCI MATERIALS TECH
Method for Producing Difluorophosphate, Non-Aqueous Electrolyte for Secondary Cell and Non-Aqueous Electrolyte Secondary Cell
ActiveUS20080305402A1Poor battery performanceExcellent coating agentPhosphorus halides/oxyhalidesNon-aqueous electrolyte accumulatorsDifluorophosphatePhysical chemistry
A difluorophosphate effective as an additive for a nonaqueous electrolyte for secondary battery is produced by a simple method from inexpensive common materials.The difluorophosphate is produced by reacting lithium hexafluorophosphate with a carbonate in a nonaqueous solvent. The liquid reaction mixture resulting from this reaction is supplied for providing the difluorophosphate in a nonaqueous electrolyte comprising a nonaqueous solvent which contains at least a hexafluorophosphate as an electrolyte lithium salt and further contains a difluorophosphate. Also provided is a nonaqueous-electrolyte secondary battery employing this nonaqueous electrolyte.
Owner:MU IONIC SOLUTIONS CORP +1
High-voltage rate electrolyte with high-and-low temperature performance and lithium ion battery using electrolyte
PendingCN107706455AAvoid decompositionImprove high temperature storage performanceSecondary cellsOrganic electrolytesHigh temperature storageLithium-ion battery
The invention discloses a high-voltage rate electrolyte with high-and-low temperature performance and a lithium ion battery using the electrolyte. The electrolyte comprises a non-aqueous solvent, a lithium salt dissolved in the non-aqueous solvent and additives, wherein the non-aqueous solvent comprises propylene carbonate (PC) and linear carboxylic ester; and the additives comprise citraconic anhydride, lithium difluorophosphate (LiPO<2>F<2>), fluoroethylene carbonate, ethylene sulfate and 1, 2-di(2-cyanoethoxyl)ethane. By applying the synergistic effect generated by the solvent system and the additive optimization combination to the lithium ion battery, excellent cycle life, low-temperature discharge characteristic and high-temperature storage characteristic of the battery still can be maintained at high-voltage rate.
Owner:EVE ENERGY CO LTD
Lithium difluorophosphate, electrolytic solution containing lithium difluorophosphate, process for producing lithium difluorophosphate, process for producing nonaqueous electrolytic solution, nonaqueo
ActiveCN101507041AEasy and efficient to prepareHigh purityCell electrodesLi-accumulatorsElectrolytic agentElectrical battery
A difluorophosphate salt, which is expensive and not readily available, can be produced with a high purity readily and efficiently from inexpensive and readily available materials. A nonaqueous electrolyte secondary battery that exhibits low-temperature discharge and heavy-current discharge characteristics and high-temperature preservation and cycle characteristics without impairing the battery safety. A hexafluorophosphate salt is reacted with a compound having a bond represented by the following formula (1) in the molecule: Si-O-Si (1). A nonaqueous electrolyte used for nonaqueous electrolyte secondary batteries including a negative electrode and a positive electrode that can occlude and discharge ions, and a nonaqueous electrolyte is prepared from a mixture obtained by mixing at least one nonaqueous solvent, a hexafluorophosphate salt and a compound having a bond represented by the following formula (1), and removing low-boiling compounds newly formed in the system, the low-boiling compounds having a lower boiling point than that of the compound having the bond represented by the formula (1): Si-O-Si (1).
Owner:MITSUBISHI CHEM CORP +1
Lithium secondary batteries and nonaqueous electrolyte for use in the same
InactiveCN101894974AExcellent low temperature discharge characteristicsCell electrodesLi-accumulatorsElectrolytic agentSilane compounds
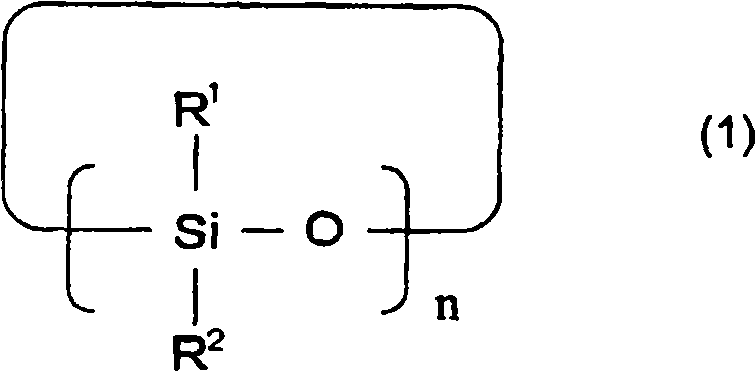
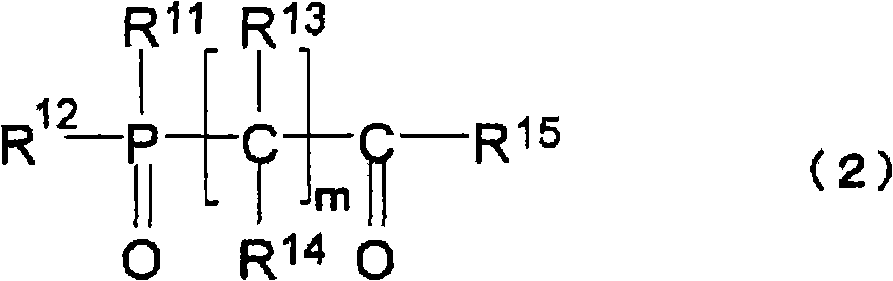
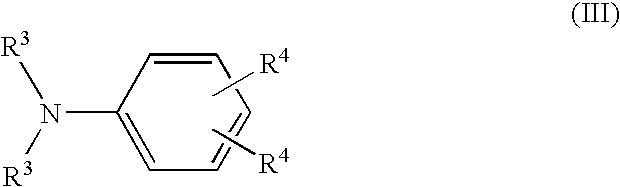
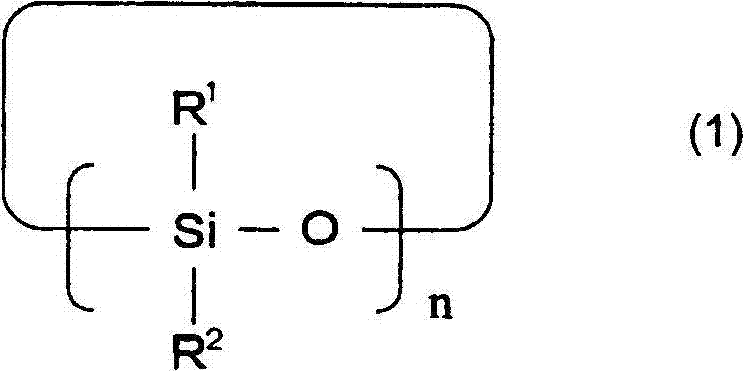
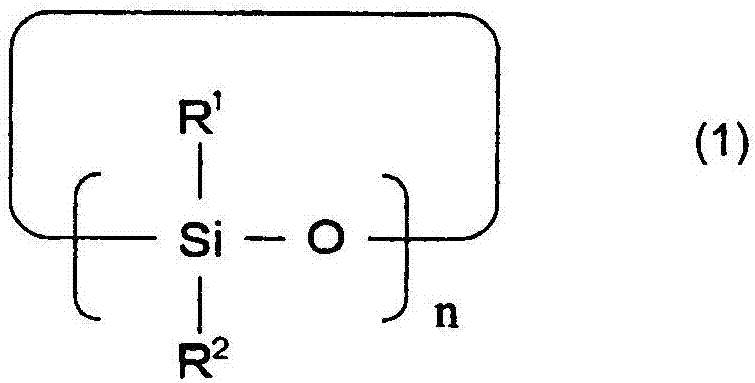
A lithium secondary battery comprising: a positive electrode and a negative electrode which each has a specific composition and specific properties; and a nonaqueous electrolyte which contains a cyclic siloxane compound represented by general formula (1), fluorosilane compound represented by general formula (2), compound represented by general formula (3), compound having an S-F bond in the molecule, nitric acid salt, nitrous acid salt, monofluorophosphoric acid salt, difluorophosphoric acid salt, acetic acid salt, or propionic acid salt in an amount of 10 ppm or more of the whole nonaqueous electrolyte. This lithium secondary battery has a high capacity, long life, and high output. [In general formula (1), R1 and R2 are an organic group having 1-12 carbon atoms and n is an integer of 3-10. In general formula (2), R3 to R5 are an organic group having 1-12 carbon atoms; x is an integer of 1-3; and p, q, and r each are an integer of 0-3, provided that 1<=p+q+r<=3. In general formula (3), R6 to R8 are an organic group having 1-12 carbon atoms and symbol A is a group constituted of H, C, N, O, F, S, Si, and / or P.
Owner:MITSUBISHI CHEM CORP
Nonaqueous electrolyte for secondary battery and nonaqueous-electrolyte secondary battery employing the same
ActiveUS20130011728A1Reduce impactLower performance requirementsCell electrodesLi-accumulatorsHigh current densityChemical structure
Owner:MU IONIC SOLUTIONS CORP
Method for producing difluorophosphate, nonaqueous electrolyte solution, and nonaqueous electrolyte secondary battery
ActiveCN102036912AEasy to manufactureHigh purityFinal product manufactureLi-accumulatorsHydrogen fluorideAlkaline earth metal
A method for producing a high-purity difluorophosphate by simple processes; a method for producing an electrolyte solution using a difluorophosphate obtained by the method; an electrolyte solution; and a secondary battery. Specifically disclosed is a method for producing a difluorophosphate, which comprises the following step (1) or (2). (1) A step wherein (A) at least one substance selected froma group consisting of phosphorus oxo acids, oxo acid anhydrides and oxyhalides is reacted with (B) a hexafluorophosphate in the presence of hydrogen fluoride. (2) A step wherein at least one halide selected from a group consisting of alkali metal halides, alkaline earth metal halides, aluminum halides and onium halides is reacted with difluorophosphoric acid in the presence of a hexafluorophosphate. Also disclosed are a nonaqueous electrolyte solution containing the thus-obtained difluorophosphate, and a nonaqueous electrolyte secondary battery comprising the nonaqueous electrolyte solution.
Owner:STELLA CHEMIFA CORP +2
Preparation method of difluoro-lithium phosphate and lithium ion battery non-aqueous electrolyte
ActiveCN104445133AHigh purityEasy to purifySecondary cellsPhosphorus compoundsHydrogen fluoridePyrophosphate
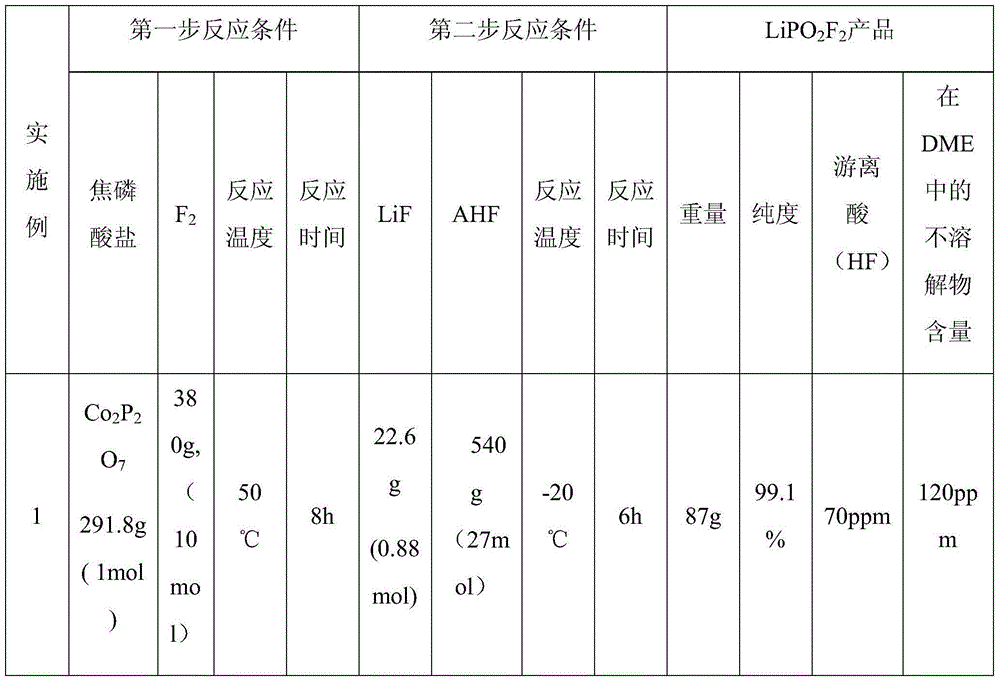
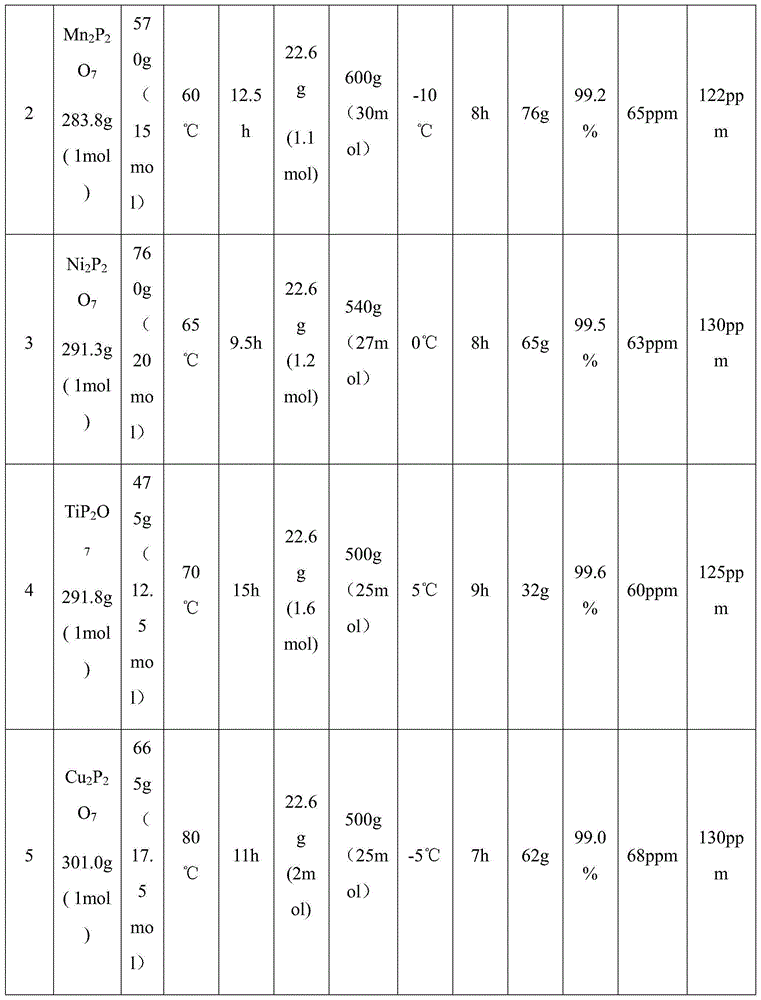
The invention provides a preparation method of difluoro-lithium phosphate. The preparation method comprises the following steps: reacting pyrophosphate with fluorine to generate a mixed gas; introducing the obtained mixed gas into an anhydrous hydrogen fluoride solution of lithium fluoride for reaction, and crystallizing, filtering and drying after the reaction to obtain a difluoro-lithium phosphate product. The invention also provides a lithium ion battery non-aqueous electrolyte containing the difluoro-lithium phosphate prepared by the method. The preparation method provided by the invention has the advantages of simple process, low cost and high product purity.
Owner:ZHEJIANG KAISN FLUOROCHEM
Silicon carbon lithium ion battery electrolyte and silicon carbon lithium ion battery using same
InactiveCN109546218AAvoid breakingImprove cycle performanceSecondary cellsOrganic electrolytesPhosphateSulfate
A silicon carbon lithium ion battery electrolyte and a silicon carbon lithium ion battery using the same belong to the field of lithium ion battery materials. The electrolyte comprises a film-formingadditive accounting for 8% to 15% by weight of the total weight of the electrolyte. The film-forming additive comprises a phosphate ester / phosphite ester compound, fluoroethylene carbonate (FEC), ethylene sulfate (DTD) and lithium difluorophosphate (LiPO2F2). The electrolyte has excellent cycle performance. The phosphate ester / phosphite ester compound has chemical structural formulas shown by theformulas (I) and (II) which are described in the specifications.
Owner:ZHUHAI COSMX BATTERY CO LTD
Nonaqueous electrolyte solution and nonaqueous electrolyte battery
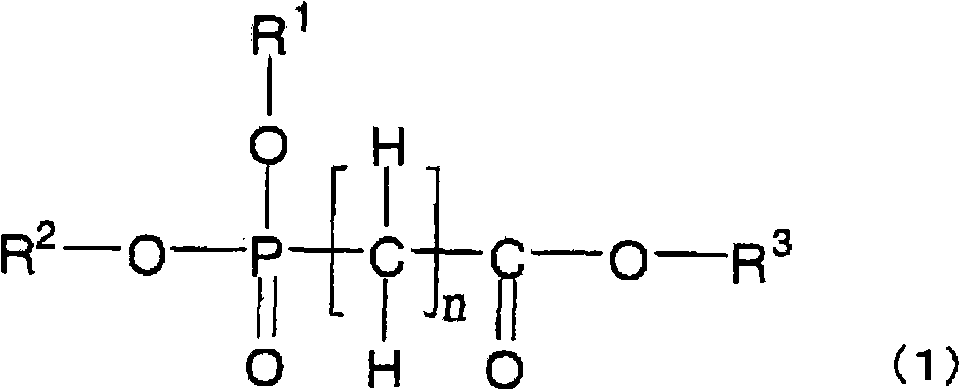
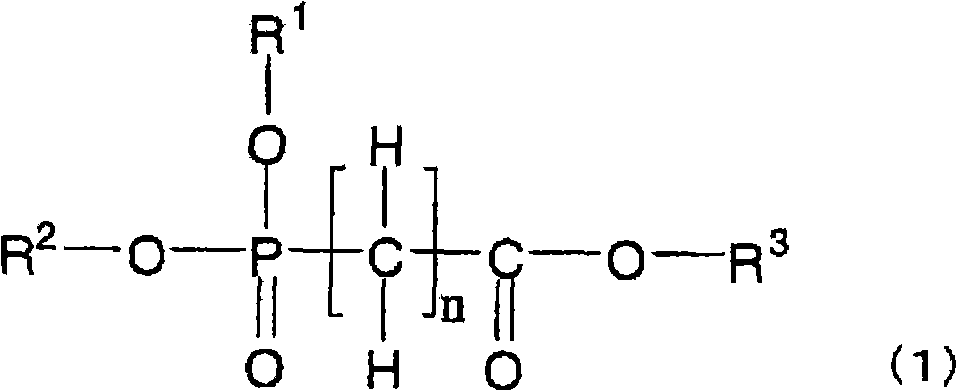
Disclosed is a nonaqueous electrolyte solution which enables to obtain a battery with high capacity which generates only a small amount of gas, while being excellent in storage characteristics and cycle characteristics. This nonaqueous electrolyte solution contains an electrolyte and a nonaqueous solvent dissolving the electrolyte, and further contains a compound represented by the general formula (1) below in an amount of not less than 0.001% by volume but less than 1% by volume in the nonaqueous solvent. Alternatively, the nonaqueous electrolyte solution contains the compound represented by the general formula (1) below in an amount of not less than 0.001% by volume but less than 5% by volume in the nonaqueous solvent, and further contains at least one compound selected from the group consisting of cyclic carbonate compounds having a carbon-carbon unsaturated bond, cyclic carbonate compounds having a fluorine atom, monofluorophosphates and difluorophosphates. In the general formula (1), R<1>-R<3> independently represent an alkyl group having 1-12 carbon atoms which may be substituted by a halogen atom; and n represents an integer of 0-6.
Owner:MITSUBISHI CHEM CORP
Method for preparing high-purity lithium difluorophosphate by utilization of organo tin fluoride
The invention discloses a method for preparing high-purity lithium difluorophosphate by the utilization of organo tin fluoride. By the utilization of the characteristic that organo tin fluoride is easily subjected to a halogen exchange reaction with a phosphorus compound containing chlorine, bromine and iodine, lithium dichlorophosphate is firstly prepared by a simple method, and then organo tin fluoride and lithium dichlorophosphate undergo a contact reaction so as to generate lithium difluorophosphate. The raw materials provided by the invention are easily available and the reactions are easy to operate. Conditions of the whole reaction process are mild; no by-products appear; the process is simple; requirements on equipment and environmental protection are low; and the possibility of impurity introduction is avoided. Thus, the lithium difluorophosphate generated by the preparation method has high purity and good quality.
Owner:中山市华玮新能源科技有限公司 +2
Non-aqueous electrolyte for battery and non-aqueous electrolyte battery comprising the same
InactiveUS20080020285A1Improve battery performanceMaintain performanceCell electrodesOrganic electrolyte cellsPhysical chemistryAniline
A non-aqueous electrolyte for a battery comprises a non-aqueous solvent containing a specified cyclic phosphazene compound and a specified difluorophosphate compound, a specified aniline derivative and a support salt.
Owner:BRIDGESTONE CORP
Lithium secondary cell and nonaqueous electrolytic solution for use therein
ActiveCN102931434AExcellent low temperature discharge characteristicsElectrode thermal treatmentFinal product manufacturePropanoic acidHyponitrite
A lithium secondary cell which comprises a positive electrode and a negative electrode which have a specific composition and specific properties and a nonaqueous electrolytic solution containing a cyclic siloxane compound represented by the formula (1), a fluorosilane compound represented by the formula (2), a compound represented by the formula (3), a compound having an S-F bond, a nitric acid salt, a nitrous acid salt, a monofluorophosphoric acid salt, a difluorophosphoric acid salt, an acetic acid salt, or a propionic acid salt in an amount of at least 10 ppm of the whole nonaqueous electrolytic solution. The lithium secondary cell has a high capacity, long life, and high output. In the general formula (1), R<1> and R<2> each is a C1-12 organic group; and n is an integer of 3-10. In the general formula (2), R<3> to R<5> each is a C1-12 organic group; x is an integer of 1-3; and p, q, and r each is an integer of 0-3, provided that 1=p+q+r=3. In the general formula (3), R<6> to R<8> each is a C1-12 organic group; and A is a group constituted of H, C, N, O, F, S, Si, and / or P.
Owner:MITSUBISHI CHEM CORP +1
Secondary battery, electrolytic solution, battery pack, electronic device, and electrical vehicle
ActiveUS20130052543A1Good chemical stabilityImprove featuresAlkaline accumulatorsOrganic electrolyte cellsImideCarboxylic acid
A secondary battery capable of improving cycle characteristics, conservation characteristics, and load characteristics is provided. The secondary battery includes a cathode, an anode, and an electrolytic solution. A separator provided between the cathode and the anode is impregnated with an electrolytic solution. The electrolytic solution includes one or more of a dicarbonic ester compound, a dicarboxylic compound, a disulfonic compound, a monofluoro lithium phosphate, and difluoro lithium phosphate and one or more of fluorinated lithium phosphate, fluorinated lithium borate, and imide lithium.
Owner:MURATA MFG CO LTD
Non-aqueous electrolyte and lithium ion battery
ActiveCN110911753ALower impedanceImprove power performanceSecondary cellsOrganic electrolytesHigh temperature storageElectrolytic agent
The invention provides a non-aqueous electrolyte and a lithium ion battery. The non-aqueous electrolyte comprises a solvent, an electrolyte lithium salt and a functional additive, and the functional additive comprises pentafluorophenyl vinyl sulfonate, 3-trifluoromethyl-5-methoxybenzonitrile and lithium difluorophosphate. When the electrolyte disclosed by the invention is applied to the lithium ion battery of a nickel-cobalt-manganese ternary material / graphite system, the high-voltage resistance of the lithium ion battery is improved, and the rate capability, the normal-temperature cycle, thehigh-temperature cycle and the high-temperature storage performance of the lithium ion battery are remarkably improved.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
Composite lithium ion battery electrolyte and lithium ion battery comprising same
ActiveCN109361017AImprove cycle performanceInhibit side effectsSecondary cellsOrganic electrolytesMethyl carbonateLithium-ion battery
The invention discloses composite lithium ion battery electrolyte and a lithium ion battery comprising the same. The composite lithium ion battery electrolyte comprises an organic solvent, lithium salt and an additive. The organic solvent is at least two of propylene carbonate (PC), ethyl methyl carbonate (EMC), dimethyl carbonate (DMC), dipropyl carbonate (DPC), allyl ethyl carbonate (AEC), allylmethyl carbonate (AMC) and the like; and the additive is selected from at least two of unsaturated carbonate, sulfur-containing organic matters, lithium borate dioxalate, lithium difluorophosphate and fluoro-imide salt. For the respective physical and chemical characteristics of the organic solvent and the additive, the types of the additive are screened and combined, by adjustment on an electrolyte additive, the ratio by which respective advantages can be played and respective shortcomings can also be suppressed is found out, the high-voltage capacity of the battery is improved, and the cycle life of the battery is prolonged.
Owner:산산어드밴스드머테리얼스(취저우)컴퍼니리미티드
Lithium ion battery electrolytic solution and lithium ion battery
InactiveCN111653829AImprove high temperature gas production performanceGood high-rate charging performanceSecondary cellsOrganic electrolytesElectrolytic agentPropane sultone
The invention discloses a lithium ion battery electrolytic solution, which comprises a film-forming additive, wherein the film-forming additive comprises vinylene carbonate, ethylene sulfate, 1,3-propane sultone and lithium difluorophosphate, wherein the vinylene carbonate accounts for 0.2%-3% of the total mass of the electrolytic solution, the ethylene sulfate accounts for 0.5%-5% of the total mass of the electrolytic solution, the 1,3-propane sultone accounts for 0.2%-3% of the total mass of the electrolytic solution, and the lithium difluorophosphate accounts for 0.2%-2% of the total mass of the electrolytic solution. According to the invention, vinylene carbonate and ethylene sulfate in the electrolytic solution form a stable and compact SEI film on the surface of the negative electrode of the battery in the formation, charging and discharging processes, and 1,3-propane sultone and lithium difluorophosphate form a passive film on the surface of the positive electrode, so that the high-temperature gas production performance of the battery is improved, the damage to the negative electrode due to dissolution of positive electrode transition metal elements in the quick charge cycleprocess is inhibited, the film forming structure of the positive electrode and the negative electrode can be improved, the side reaction is reduced, and the battery is ensured to have good high-ratecharging performance and cycle life.
Owner:CHINA AVIATION LITHIUM BATTERY RES INST CO LTD +1
Method for preparing lithium difluorophosphate
The invention discloses a method for preparing lithium difluorophosphate. The method comprises the following steps: dissolving lithium carbonate and lithium hexafluorophosphate in an aprotic solvent, uniformly mixing above substances and the solvent, filling a closed container with the obtained solution, carrying out a high-temperature and high-pressure reaction for a certain time, carrying out rotary evaporation to remove the solvent, and purifying the obtained reaction product through a washing process to obtain highly-pure lithium difluorophosphate. The method for preparing the lithium difluorophosphate through adopting a solvothermal technology has the advantages of realization of high-temperature rapid preparation of the lithium difluorophosphate, small dosage of the solvent, high easiness in purification, and very high purity of the target product.
Owner:SUN YAT SEN UNIV +1
Non-aqueous electrolyte for battery and non-aqueous electrolyte battery comprising the same
InactiveUS20080020276A1High flame retardanceSufficient battery performanceOrganic electrolyte cellsSecondary cellsSolventNon aqueous electrolytes
A non-aqueous electrolyte for a battery comprises a non-aqueous solvent containing a specified cyclic phosphazene compound and a specified difluorophosphate compound, a dicarboxylic anhydride compound having a cyclic structure and a support salt.
Owner:BRIDGESTONE CORP
Method for preparing lithium difluorophosphate
ActiveCN107720717AReduce utilizationIncrease profitPhosphorus compoundsLithium hydroxideLithium carbonate
The invention discloses a method for preparing lithium difluorophosphate. The synthesis principle is that difluorophosphate is subjected to a contact reaction with lithium hydroxide in a non-aqueous solvent, or difluorophosphate contacts is subjected to the contact reaction with lithium carbonate in the non-aqueous solvent, and specifically a reaction equation is that HPO2F2+LiOH=LiPO2F2+H2O, or 2HPO2F2+Li2CO3=2LiPO2F2+H2O+CO2. According to the method, difluorophosphate and lithium carbonate or lithium hydroxide are adopted as main raw materials, lithium hexafluorophosphate which is common inthe prior art is not used, and thus the reaction cost is reduced; in addition, in the preparation process, no harmful gas such as a fluorine gas is used, and the reaction process is easy to control, high in security coefficient and low in equipment cost; the reaction process is a solid-liquid reaction, the raw materials are target products are both free of gas, the raw materials and the products are easy in purity control and convenient to transport, and the raw material utilization rate is high, so that the cost can be lowered.
Owner:TIANJIN JINNIU POWER SOURCES MATERIAL +1
Lithium secondary cell and nonaqueous electrolytic solution for use therein
InactiveCN107069091AExcellent low temperature discharge characteristicsFinal product manufactureActive material electrodesLithiumAcetic acid
A lithium secondary cell which comprises a positive electrode and a negative electrode which have a specific composition and specific properties and a nonaqueous electrolytic solution containing a cyclic siloxane compound represented by the formula (1), a fluorosilane compound represented by the formula (2), a compound represented by the formula (3), a compound having an S-F bond, a nitric acid salt, a nitrous acid salt, a monofluorophosphoric acid salt, a difluorophosphoric acid salt, an acetic acid salt, or a propionic acid salt in an amount of at least 10 ppm of the whole nonaqueous electrolytic solution. The lithium secondary cell has a high capacity, long life, and high output. In the general formula (1), R<1> and R<2> each is a C1-12 organic group; and n is an integer of 3-10. In the general formula (2), R<3> to R<5> each is a C1-12 organic group; x is an integer of 1-3; and p, q, and r each is an integer of 0-3, provided that 1=p+q+r=3. In the general formula (3), R<6> to R<8> each is a C1-12 organic group; and A is a group constituted of H, C, N, O, F, S, Si, and / or P.
Owner:MITSUBISHI CHEM CORP +1
Method for producing difluorophosphate
ActiveCN104114487AEasy to manufactureHigh purityFinal product manufactureLi-accumulatorsAlkaline earth metalPhosphate
Provided is a method for producing difluorophosphate which is characterized by obtaining the difluorophosphate by reacting at least one salt, as a raw material, selected from a halide salt, a carbonate, a phosphate, a hydroxide and an oxide of an alkali metal, an alkali earth metal or an onium with a difluorophosphoric acid in the difluorophosphoric acid, then separating a precipitate from the difluorophosphoric acid by solid liquid separation, the precipitate being precipitated by crystallization operation in the difluorophosphoric acid, and distilling the difluorophosphoric acid contained in the precipitate.
Owner:STELLA CHEMIFA CORP
Preparation method of lithium phosphate difluoride
The invention relates to a preparation method of lithium phosphate difluoride, in particular to a method for producing industrially and economically-superior lithium phosphate difluoride for producingnonaqueous electrolyte batteries. The preparation method of the lithium phosphate difluoride at least comprises the following steps: (1) performing a reaction on lithium halide, phosphorus pentahalide and lithium hexafluorophosphate to obtain tetra-halogen lithium phosphate difluoride; (2) making tetra-halogen lithium phosphate difluoride react with a substance containing active oxygen to obtainlithium phosphate difluoride.
Owner:如鲲(山东)新材料科技有限公司
Preparation method of high purity difluorophosphate
ActiveCN105236380AWide variety of sourcesLow costPhosphorus compoundsHydrogen fluorideAlkaline earth metal
The invention discloses a preparation method of high purity difluorophosphate. The preparation method comprises the following steps: dissolving meta-phosphoric acid in alcohol, then introducing hydrogen fluoride into the alcohol to carry out fluorination reactions to obtain monofluorophosphoric acid, reacting monofluorophosphoric acid with phosphorus oxyfluoride gas to obtain difluorophosphoric acid, then reacting difluorophosphoric acid with alkali metals or alkali-earth metal halides, and separating the reaction products to obtain high purity difluorophosphate. The preparation method has the advantages that the raw materials are cheap and easily-available, a homogenous reaction technology is adopted, thus the reaction speed is greatly increased; during the reaction process, the excess raw materials and solvents can be separated, recovered, and reused through fractionation, thus the amount of generated wastes is reduced; and during the whole process, no water is generated, so the product will not be hydrolyzed. The high purity difluorophosphate can be used to produce electronic chemicals.
Owner:GUANGZHOU TINCI MATERIALS TECH +1
Preparation method of difluorophosphoric acid alkali metal salt
ActiveCN105236368AEnsure safetyImprove securityPhosphorus halides/oxyhalidesElectrolytic agentPhosphate
The invention discloses a preparation method of difluorophosphoric acid alkali metal salt. In the method, the difluorophosphoric acid alkali metal salt is produced through a reaction of hexafluorophosphates of alkali metals and corresponding phosphates of the alkali metals in a solid phase or in a non-aqueous solvent, wherein the reaction equation is MPF6+M3PO4=2MPO2F2+2MF (M=Li, Na, K, Rb, Cs), the molar ratio of MPF6 to M3PO4 is 0.8-1.5:1, preferably 1.1-1.2:1, namely, the hexafluorophosphates of alkali metals is excessive properly to ensure reaction of the phosphates to be carried completely. The method can be used for preparing the difluorophosphoric acid alkali metal salt under safe situations, thereby improving safety of the preparation process and ensuring safety of preparation operators. The difluorophosphoric acid alkali metal salt is produced through a reaction of the hexafluorophosphates and the phosphates of the alkali metals, and the target product can be produced whether the reaction is carried out in solid phases or liquid phases under mild conditions without dangerous gas raw materials and generation of acidic gas or water which may damage performances of an electrolyte.
Owner:TIANJIN JINNIU POWER SOURCES MATERIAL +1
Electrolyte functional additive for lithium ion battery, lithium ion battery electrolyte and lithium ion battery
InactiveCN111211351AImprove high temperature storage performanceImprove high temperature cycle performanceFinal product manufactureSecondary cells servicing/maintenanceElectrolytic agentElectrical battery
The invention relates to an electrolyte functional additive for a lithium ion battery, a lithium ion battery electrolyte and a lithium ion battery, and belongs to the technical field of lithium ion batteries. The electrolyte functional additive for the lithium ion battery is prepared from the following components in parts by weight: 0.2-2 parts of vinylene carbonate; 0.5-2.5 parts of ethylene sulfate; 0.2-1 part of lithium difluorophosphate; 0.2-1 part of a film-forming agent; and 0.5-2.5 parts of fluoroethylene carbonate. The film-forming agent is at least one of tris(trimethylsilane) borateand tris(trimethylsilane) phosphate. When the electrolyte functional additive for the lithium ion battery is used for the lithium ion battery made of a high-capacity ternary positive electrode material, excellent SEI films can be formed on the surfaces of the positive electrode and negative electrode of the lithium ion battery, so that direct contact between an electrolyte and the surface of the positive electrode can be effectively prevented, meanwhile, the dissolution of metal ions is reduced, and the cycle performance of the battery is obviously improved.
Owner:CHINA AVIATION LITHIUM BATTERY LUOYANG
Non-aqueous electrolyte for lithium secondary battery and lithium secondary battery comprising same
Owner:LG ENERGY SOLUTION LTD
Lithium battery electrolyte and lithium ion battery
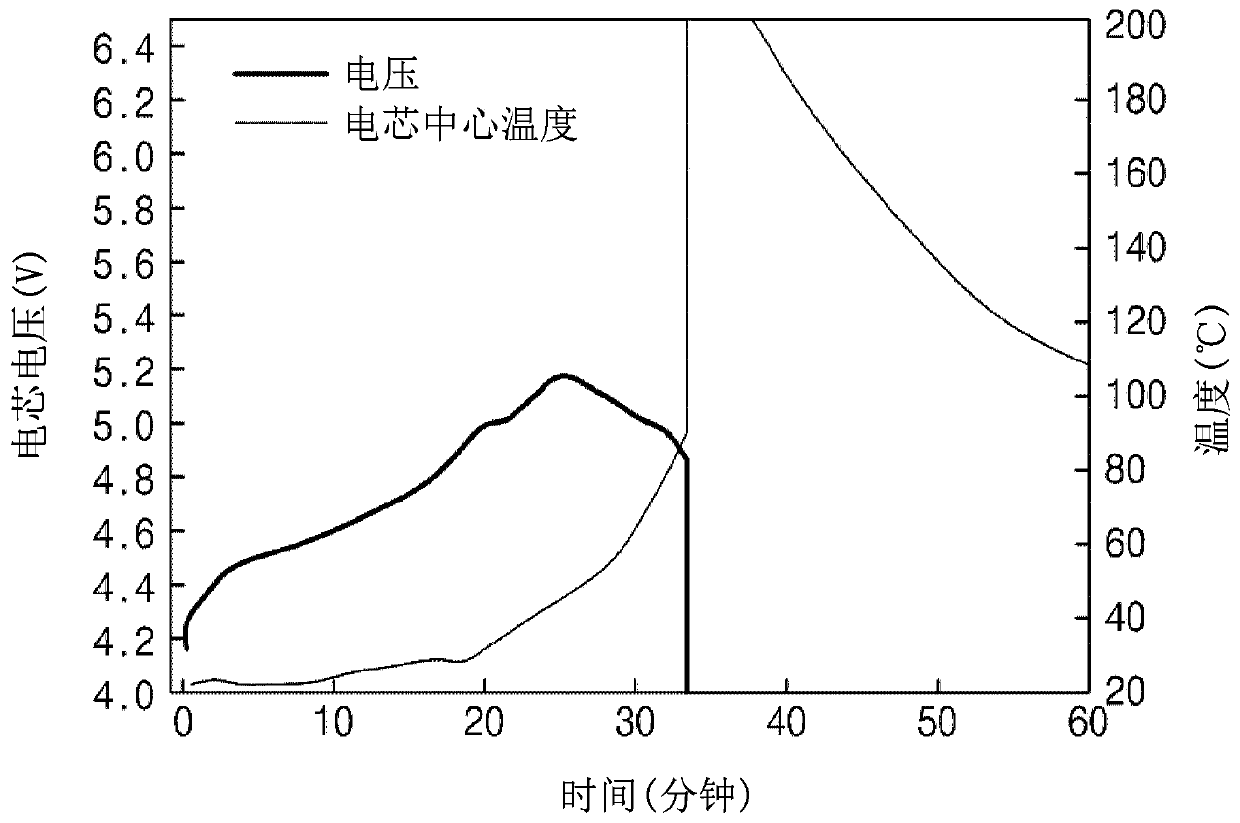
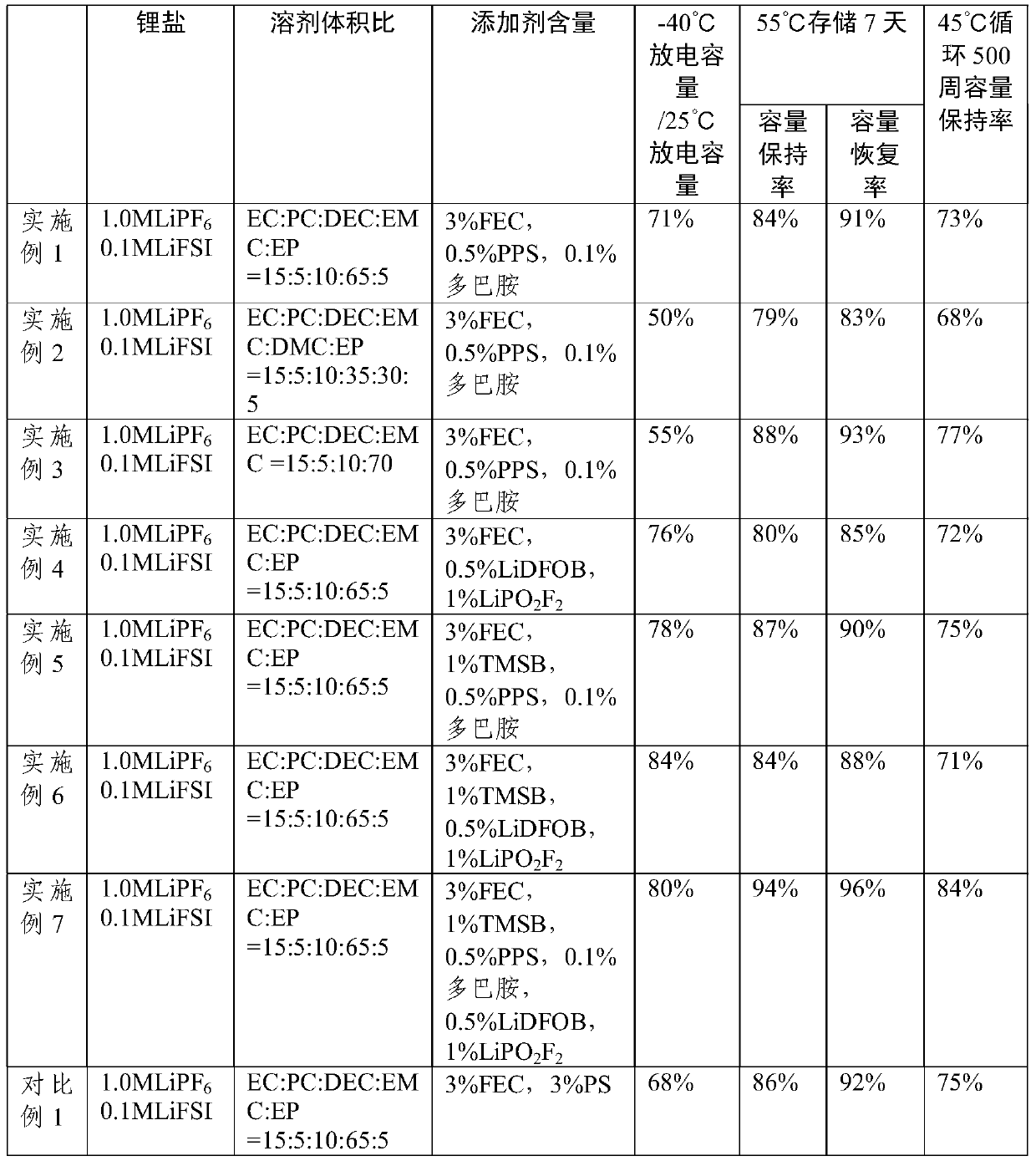
ActiveCN110994030AImprove wettabilityImprove low temperature discharge capacitySecondary cellsOrganic electrolytesElectrolytic agentOrganosolv
The invention relates to the technical field of lithium ion battery materials, in particular to a lithium battery electrolyte and a lithium ion battery. The lithium battery electrolyte comprises an organic solvent, a lithium salt and an additive, wherein the additive comprises fluoroethylene carbonate and one or more than two selected from pyridinium propanesulfonate, dopamine, lithium oxalyldifluoroborate and lithium difluorophosphate. According to the electrolyte disclosed by the invention, the pyridinium propanesulfonate, the dopamine, the lithium oxalyldifluoroborate and the lithium difluorophosphate take a synergistic effect to replace traditional propane sultone, so that the low-temperature discharge capacity is greatly improved while the high-temperature storage and high-temperaturecycle performance of the battery is improved, and harmful substances are not generated; meanwhile, ethyl acetate is adopted to improve the wettability of the electrolyte to an electrode material anda diaphragm under a low-temperature condition.
Owner:CHINA AUTOMOTIVE BATTERY RES INST CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com