Preparation method of high purity difluorophosphate
A technology of difluorophosphate and difluorophosphoric acid, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of complex separation and purification, difficulty in purification, high price of lithium hexafluorophosphate, etc., to increase reaction rate and improve production efficiency , to avoid the effect of hydrolysis problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Preparation of monofluorophosphoric acid: Add 116g of ammonium dihydrogen phosphate into an alloy kettle, heat to 400°C and react at a constant temperature for 6 hours. The decomposed gas is absorbed by water. After the reaction is completed, 80g of transparent liquid is obtained. Cool to room temperature and turn into a solid. The treated alcohol is dissolved, then the temperature is lowered, and liquid hydrogen fluoride is slowly added for fluorination, and the reaction is continued after the addition is complete. Heating and evaporating under reduced pressure to remove the alcohol, and then separating and removing the precipitated excess metaphosphoric acid to obtain a clear monofluorophosphoric acid oily liquid.
[0046] Preparation of difluorophosphoric acid: Take 80g CaF 2 (1.03mol)195gP 2 o 5 Heating to 500°C for solid phase reaction, the generated gas is condensed in a dry ice bath, the crude product is distilled and condensed twice in a dry ice bath, and the ...
specific Embodiment : Embodiment 1-6
[0051] The preparation of monofluorophosphoric acid: (yield is based on metaphosphoric acid)
[0052]
[0053] Preparation of difluorophosphoric acid: (yield is based on monofluorophosphoric acid)
[0054]
[0055] The preparation of difluorophosphate: (yield is in difluorophosphoric acid)
[0056]
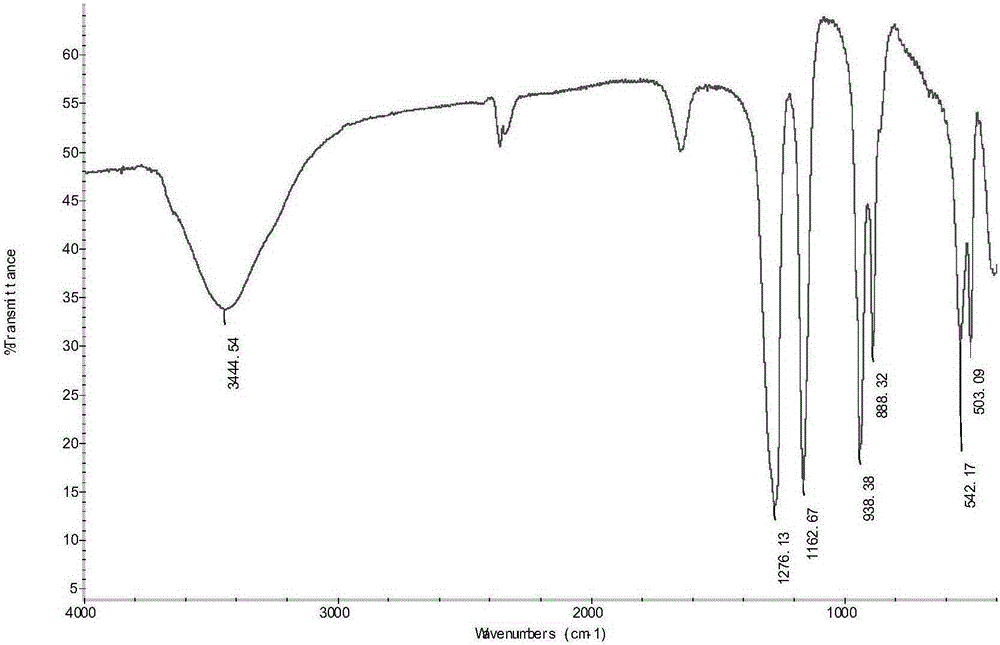
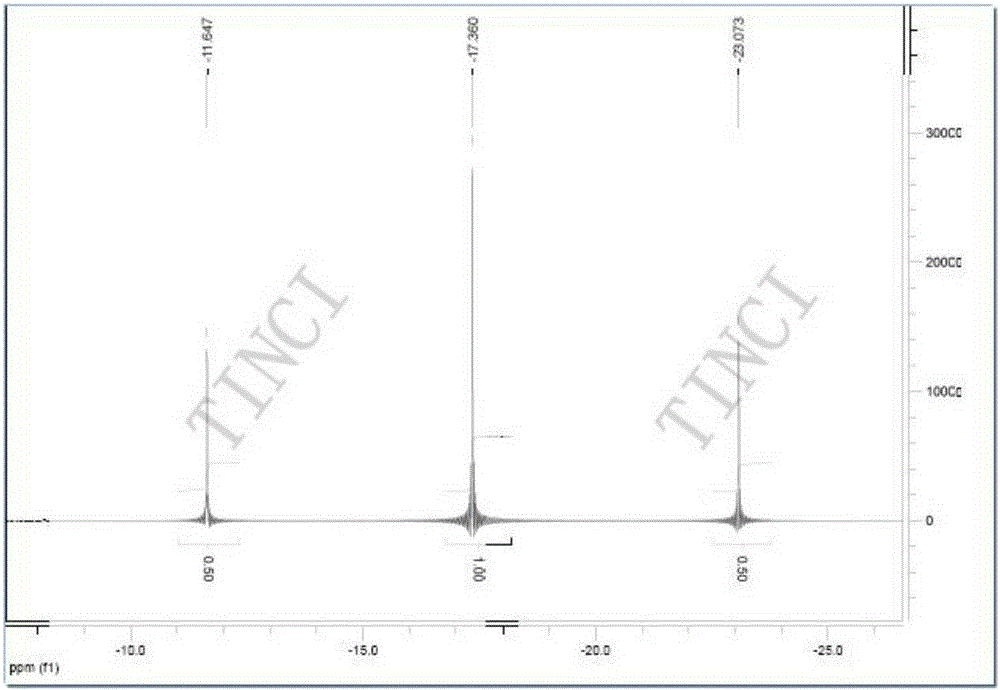
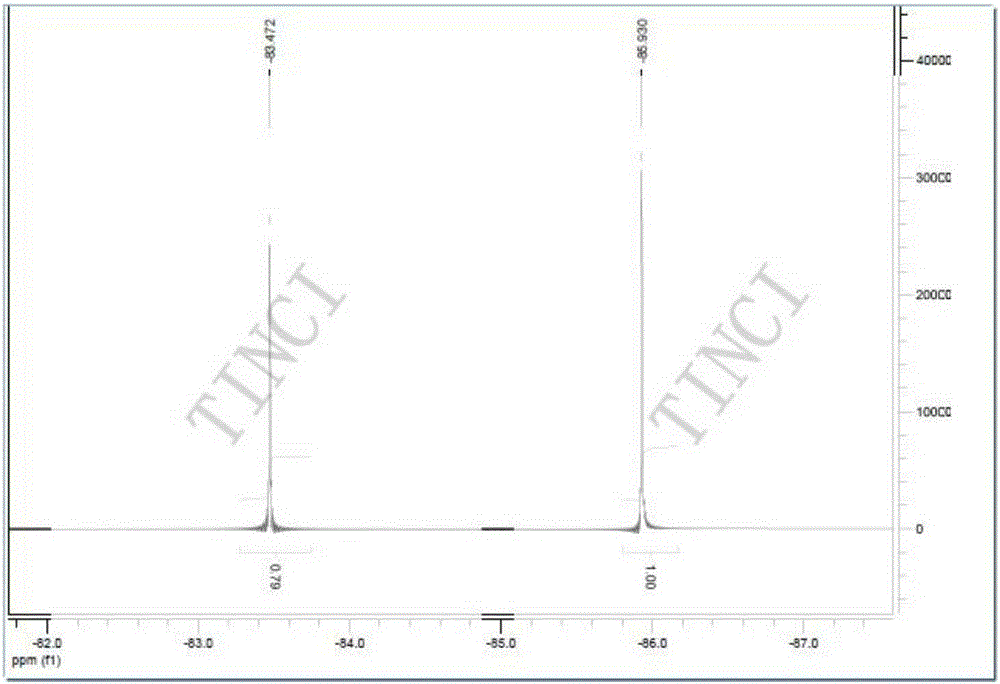
[0057] Gained difluorophosphate (for example the difluorophosphate that Example 1 obtains) adopts U.S. Thermo Company IR200 type infrared spectrometer (KBr tablet method) and Bruker Company Avance400HD type nuclear magnetic resonance spectrometer (solvent: deuterated acetone) to carry out structural confirmation (results such as Figure 1-Figure 3 As shown), the detection result is that the peak position of the sample infrared and nuclear magnetic spectrum is consistent with that of the standard, and there is no impurity peak on the nuclear magnetic spectrum. Purity was detected by Swiss Metrohm 833 ion chromatograph 2 f 2 - The anion content was calculated and the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com