Preparation method of difluoro-lithium phosphate and lithium ion battery non-aqueous electrolyte
A technology of lithium difluorophosphate and non-aqueous electrolyte, which is applied in the field of electronic chemicals, can solve problems such as complex process, many by-products, and affecting batteries, and achieve the effects of high product purity, excellent performance, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
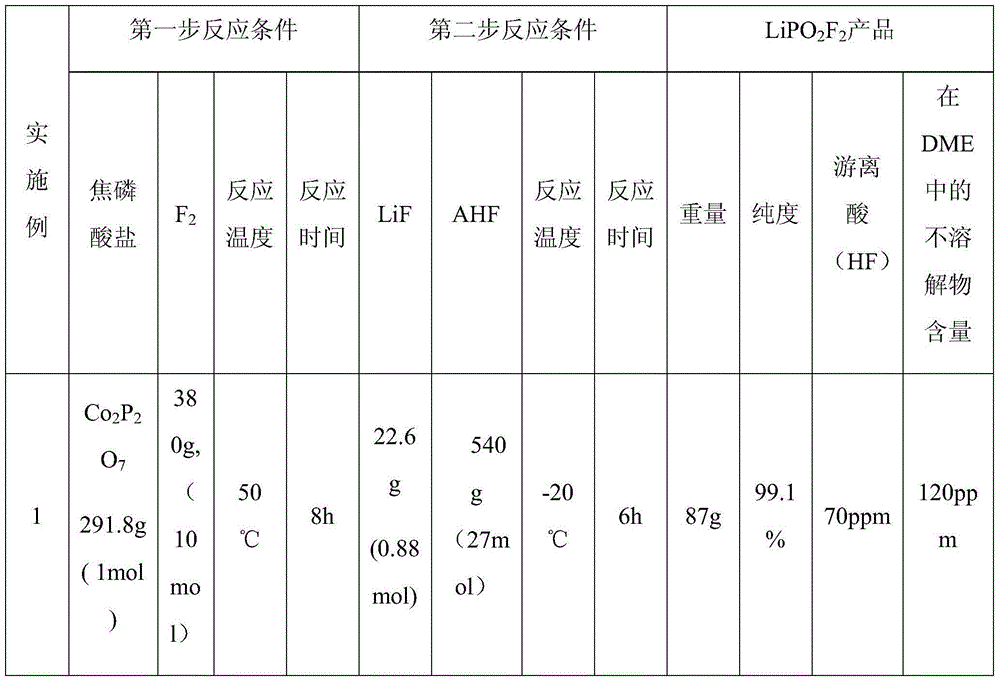
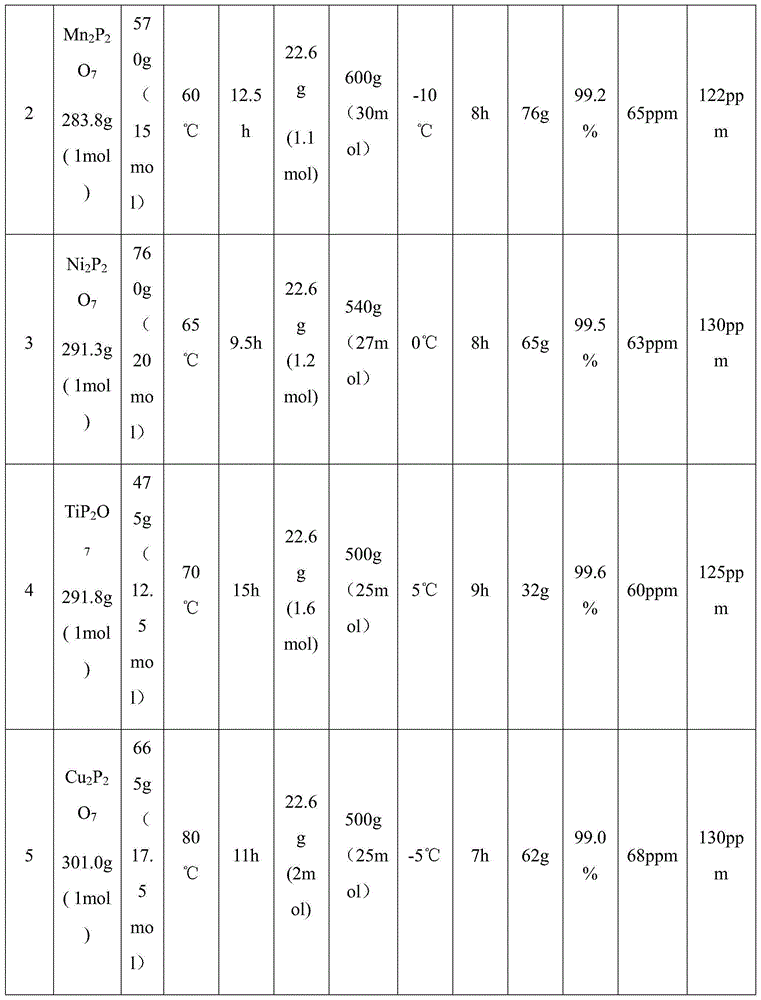
[0035] The first step reaction: under the protection of nitrogen atmosphere, first add pyrophosphate into the reactor, and then feed fluorine gas (F 2 ) to react, control the reaction temperature and the reaction time at the set value, and collect the generated gas in the gas cylinder for subsequent use. The reaction conditions are shown in Table 1.
[0036] The second step reaction: add lithium fluoride (LiF) to the reactor after nitrogen drying, control the temperature of the reactor at the set value, then pass anhydrous hydrogen fluoride solution into the reactor, start stirring to fully dissolve LiF in After the anhydrous hydrogen fluoride solution, the mixed gas obtained in the first step reaction is passed into the reactor for reaction. After the reaction, the reaction product is cooled and crystallized at -40°C, and then filtered in a nitrogen atmosphere glove box , the resulting crystals were dried at 180°C for 12 hours under the protection of nitrogen to obtain lithi...
Embodiment 6-10
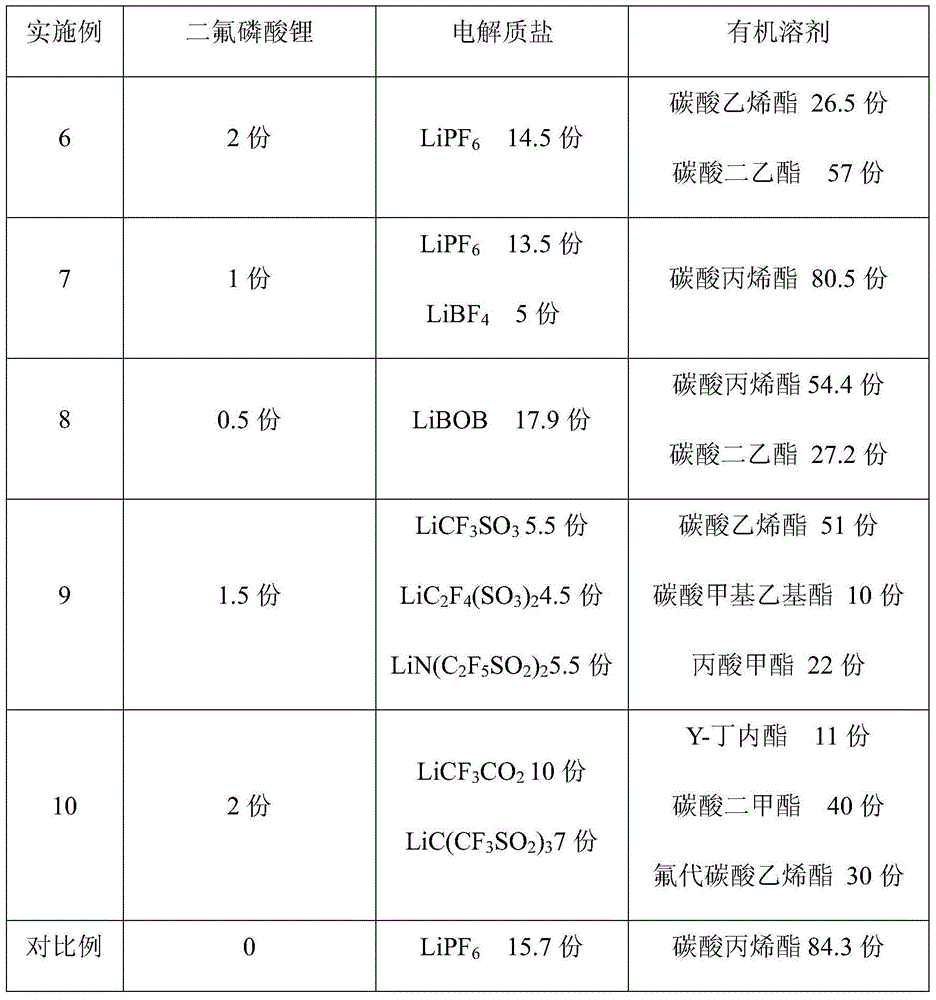
[0038] The formula of the lithium ion battery non-aqueous electrolyte of lithium difluorophosphate is shown in Table 2, and its preparation method is as follows:
[0039] According to the formula, in a dry argon-protected glove box, add the electrolyte salt to the solvent, stir evenly, and then add the lithium difluorophosphate prepared in any one of Examples 1-5 to obtain a non-aqueous lithium ion battery electrolyte.
PUM
| Property | Measurement | Unit |
|---|---|---|
| voidage | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com