Method for preparing lithium difluorophosphate
A technology of lithium difluorophosphate and difluorophosphoric acid, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as high production risk factor, high raw material utilization rate, complex production process, etc., and reduce production cost , convenient transportation, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
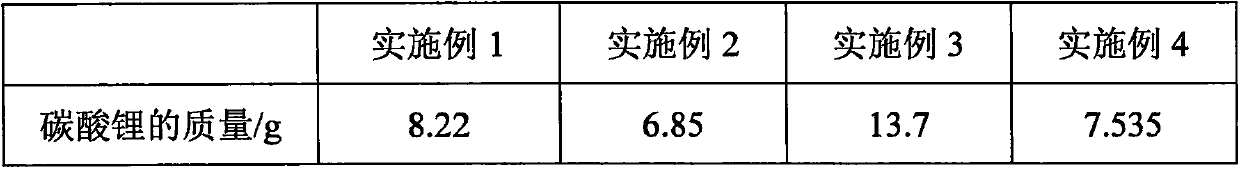
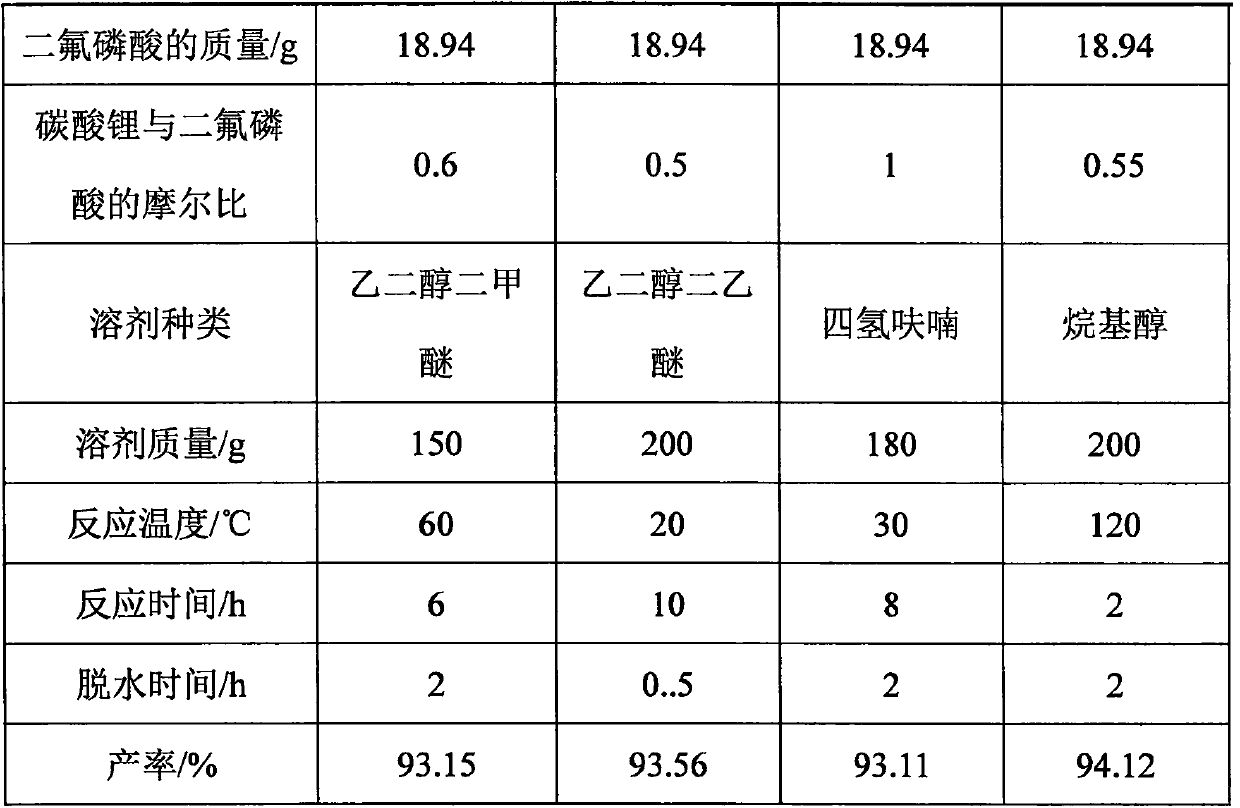
Embodiment 1-4
[0070] The reaction principle is that lithium carbonate and difluorophosphoric acid react in a benign non-aqueous solvent of lithium difluorophosphate, and the reaction equation is: 2HPO 2 f 2 +Li 2 CO 3 =2LiPO 2 f 2 +H 2 O+CO 2 .
[0071] Specific steps include:
[0072] (1), reflux reaction
[0073] 1. Take lithium carbonate and difluorophosphoric acid in proportion, and set aside; ensure that lithium carbonate is excessive, so as to avoid containing unreacted difluorophosphoric acid in the solution obtained after the completion of the reaction, thereby causing the purity of the product to decrease and the yield to decrease;
[0074] 2. Add the above-mentioned weighed lithium carbonate into an appropriate amount of benign non-aqueous solvent of lithium difluorophosphate, stir to obtain a uniform mixture; obtaining a uniform mixture can stabilize the system, and lithium carbonate can be uniformly dispersed in the solvent; The amount of solvent used will ensure that t...
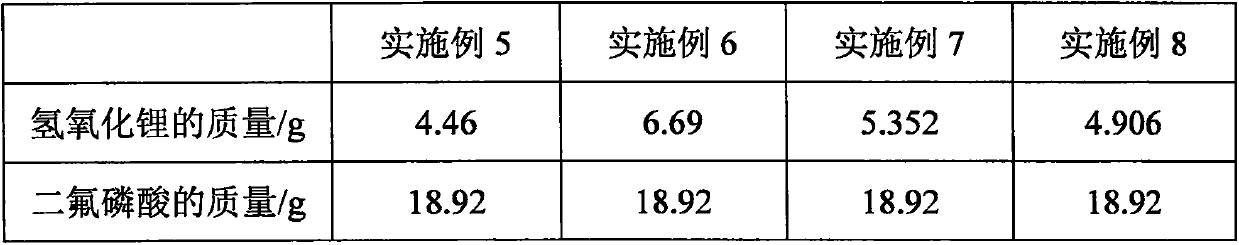
Embodiment 5-8
[0087] The reaction principle is that lithium hydroxide and difluorophosphoric acid react in a benign non-aqueous solvent of lithium difluorophosphate, and the reaction equation is: HPO 2 f 2 +LiOH=LiPO 2 f 2 +H 2 O;
[0088] Specific steps include:
[0089] (1), reflux reaction
[0090] 1. Weigh lithium hydroxide and difluorophosphoric acid in proportion, and set them aside; ensure that lithium hydroxide is excessive, so as to avoid containing unreacted difluorophosphoric acid in the solution obtained after the reaction is completed, thereby causing the purity of the product to decrease and the yield to decrease;
[0091] ②. Add the above-mentioned weighed lithium hydroxide into an appropriate amount of benign non-aqueous solvent of lithium difluorophosphate, and stir to obtain a uniform mixture; obtaining a uniform mixture can stabilize the system, and lithium hydroxide can be uniformly distributed in the solvent. Dispersion; the amount of solvent used should ensure th...
Embodiment 9-12
[0104] The reaction principle is that lithium carbonate and difluorophosphoric acid react in a poor non-aqueous solvent of lithium difluorophosphate, and the reaction equation is: 2HPO 2 f 2 +Li 2 CO 3 =2LiPO 2 f 2 +H 2 O+CO 2 .
[0105] Specific steps include:
[0106] (1) Crystallization reaction
[0107] ①. Weigh lithium carbonate and difluorophosphoric acid in proportion and set them aside; control the excess of difluorophosphoric acid to completely react the lithium carbonate in the system, so that all the insoluble solids in the final reaction system are lithium difluorophosphate, so as to ensure the stability of the product. purity;
[0108] ②. Add the above-mentioned weighed lithium carbonate into the poor non-aqueous solvent of lithium difluorophosphate, stir to obtain a uniform mixture; the amount of poor water solvent should meet the requirement of uniform dispersion of lithium carbonate in it; at the same time, due to the reaction After the end, a part of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com