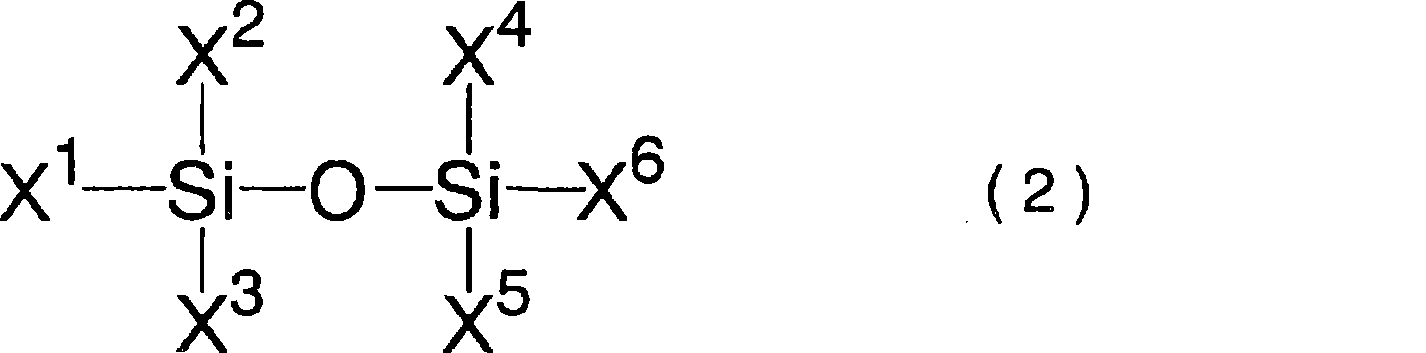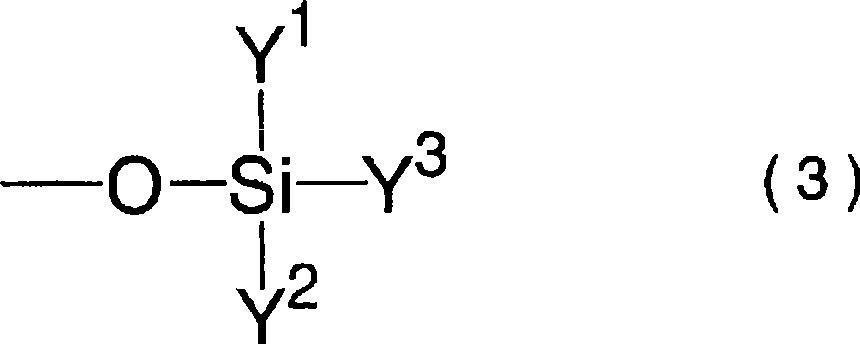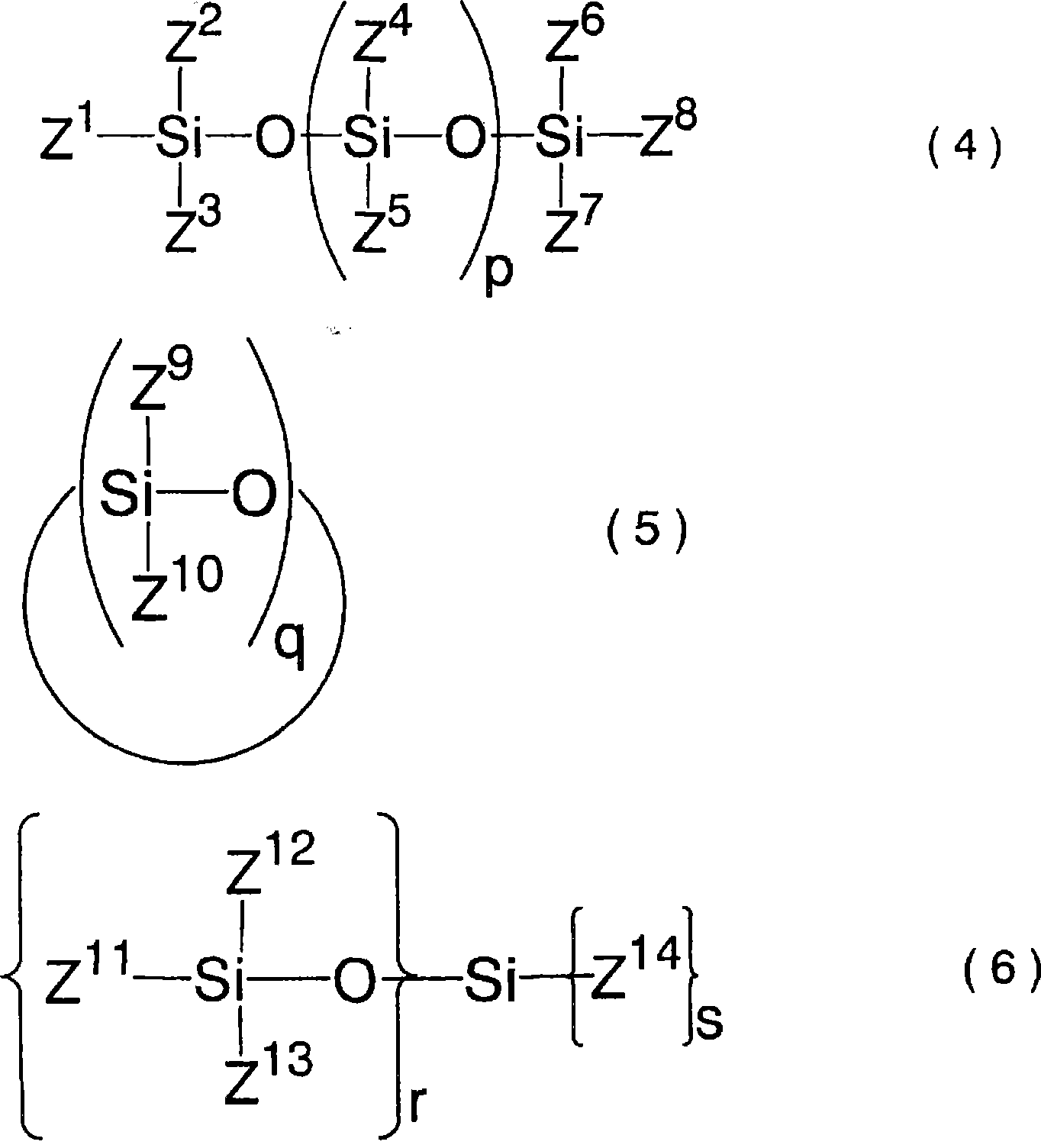Lithium difluorophosphate, electrolytic solution containing lithium difluorophosphate, process for producing lithium difluorophosphate, process for producing nonaqueous electrolytic solution, nonaqueo
A technology of lithium difluorophosphate and non-aqueous electrolyte, which is applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolytes, secondary batteries, etc., and can solve the problems of high resistance, low-temperature discharge characteristics, and high-current discharge characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0173] The preparation method of the electrolytic solution containing lithium difluorophosphate is not particularly limited as long as it does not significantly impair the effect of the present invention, but it is preferably prepared in the same way as described in "4-5. Preparation method of non-aqueous electrolytic solution". Prepare.
[0174] 《2-3. Additives》
[0175] As additives used in the electrolytic solution containing lithium difluorophosphate of the present invention, the same additives as those described in "4-4. Additives" can be used.
[0176] One kind of additives may be used alone, or two or more kinds may be used in any combination and ratio.
[0177] "2-4. With (1 / nM n+ )F - Represented Compounds》
[0178] In the electrolytic solution containing lithium difluorophosphate of the present invention, it contains 1.0×10 -2 mol Kg -1 Below (1 / nM n+ )F - .
[0179] (1 / nM n+ )F - M in is a cation other than H, and examples thereof include alkali metals, a...
Embodiment 1~23
[1512] Examples 1 to 23 will be described.
[1513]
[1514] A valve, a thermometer, a pressure gauge, and a safety valve were installed on the lid of a SUS316L airtight container with a nominal 1L (actual volume: 1.3L) as a reaction device. After the reaction device is fully dried, put it into a box filled with inert gas (nitrogen, argon, helium, etc.), add hexafluorophosphoric acid salt, solvent and specific structure compound into the reaction tank, and then put it into the tank with The stirring bar of the magnetic stirrer was covered and sealed in this state, and the reaction vessel was taken out from the box, and the reactions of Examples 1 to 23 below were carried out.
Embodiment 1~17
[1516] As Examples 1 to 17, each of the examples was reacted according to the combination of the experimental conditions described in the following Tables 1 to 3 to prepare difluorophosphate. Moreover, evaluation about each Example was performed, and the result is also shown in Table 1 - Table 3 together.
[1517] That is, in the above-mentioned reaction apparatus, the hexafluorophosphate and the specific structure compound are dissolved in a reaction solvent, and the reaction is carried out while stirring using a magnetic stirrer. In addition, in each example, the raw materials used for the reaction (hexafluorophosphate and specific structure compounds), the type and amount of the reaction solvent, and the reaction temperature and reaction time are as shown in Tables 1 to 3.
[1518] After the reaction, the reaction solvent was in the state shown in the "state after the reaction" column of Tables 1 to 3. The solid precipitated in the reaction solvent was separated according ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com