Derivative of 2-deoxy-D-ribose, preparation method and application thereof
A kind of use, ribose technology, applied in the field of 2-deoxy-D-ribose derivatives and its preparation, can solve the problems of rising cost, reducing yield, increasing feeding amount, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
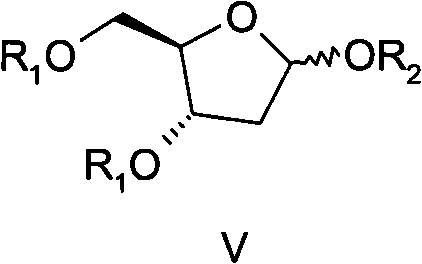
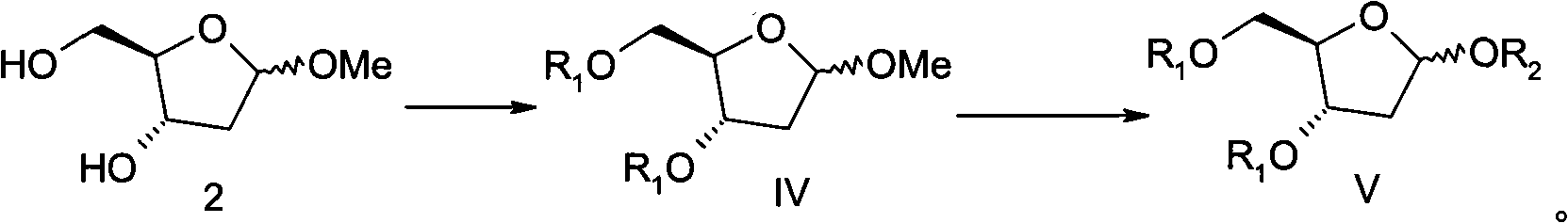
[0064] The preparation method of the compound of formula V provided by the present invention is to firstly carry out acetylation protection on the 3 and 5 hydroxyl groups of 1-O-methyl-2-deoxy-D-ribose (compound 2) to obtain the compound of formula IV, and then The 1-position methoxyl group of formula IV compound is converted into substituted acetoxyl group (OR 2 ) to obtain formula V compound:
[0065]
[0066] The preparation of compound 2 has been reported in literature (for example, Synthetic Communication, 1997, 27(20): 3505-3511); compound 2 and anhydride R 1 -O-R 1 or acid halide R 1 -X (preferably anhydride R 1 -O-R 1 ) are mixed in an organic solvent, and reacted for 10-60 hours (preferably 15-48 hours) in the presence of a deacidifying agent to obtain a compound of formula IV. The organic solvent includes various aprotic solvents. The aprotic solvent mentioned is selected from halogenated alkanes (such as dichloromethane, 1,2-ethylene dichloride, etc.), hydr...
Embodiment 1
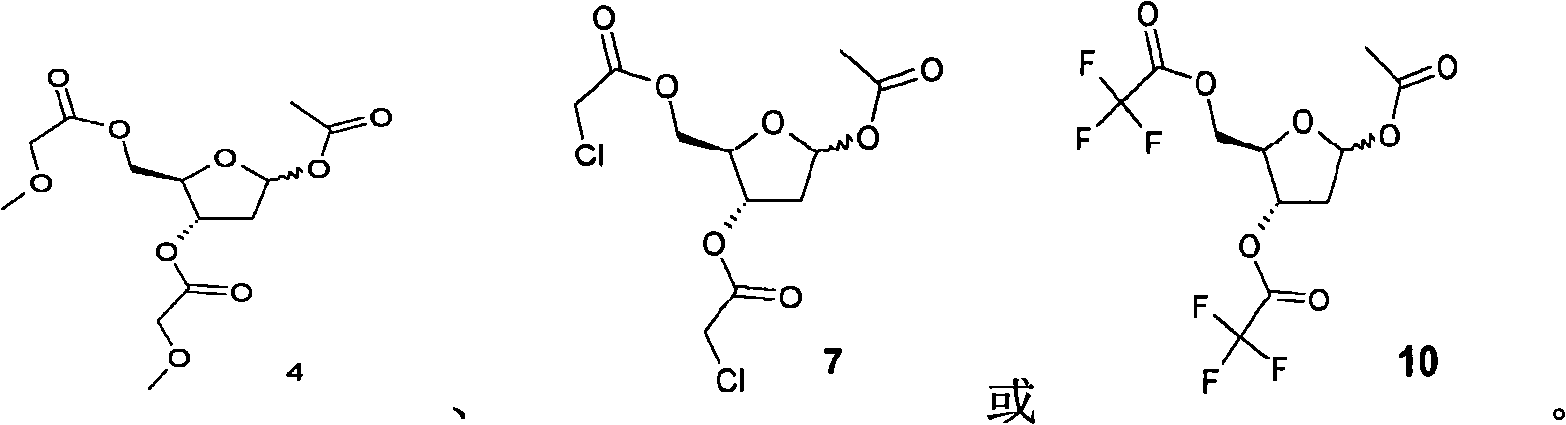
[0125] Preparation of 1-O-acetyl-3,5-di-O-methoxyacetyl-2-deoxy-D-ribose (4)
[0126]
Embodiment 11
[0128] Preparation of 1-O-methyl-2-deoxy-D-ribose (2)
[0129] Add 2-deoxy-D-ribose (1) (300 g) to methanol (3.6 L), then add 1% methanolic hydrogen chloride solution (600 ml), and stir at room temperature for 25 minutes under nitrogen protection. Sodium bicarbonate (120 g) was added and stirred for 10 minutes. filter. The obtained filtrate was concentrated under reduced pressure to obtain oil 2 (331 g), which was directly used in the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com