Preparation method of 1,1-difluoroethylene
A technology of ethylene difluoride and chloroethane, which is applied in the field of preparation of 1,1-ethylene fluoride, can solve the problems of unindustrialization of catalytic cracking, poor catalyst stability and service life, etc., achieve high purity, reduce production and Dealing with costs and preparing green effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
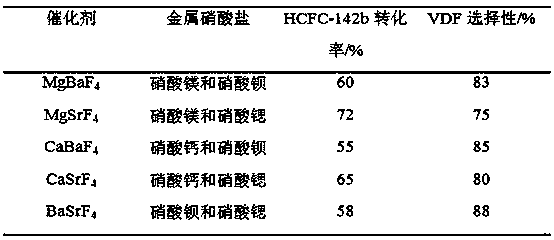
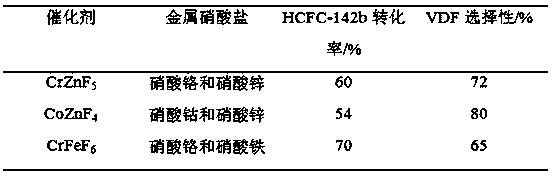
[0029] First, take 0.01 mol of each of the two transition metal nitrates and put them into a container, and add 100 mL of water. After mixing and stirring evenly, add 0.04-0.06mol solid NH 4F (according to the two metals can form precipitate required F - Calculation) to form a precipitate, and continue to stir for 2h before filtering. The solid obtained after filtration was dried at 110° C. for 12 hours, and calcined at 500° C. for 4 hours to obtain a composite catalyst of transition metal fluoride.
[0030] Then, the obtained composite catalyst catalyzes the removal of HCl from 1,1-difluoro-1-chloroethane to prepare vinylidene fluoride. The reaction is carried out in a plug-flow tube reactor. The reaction tube of the plug-flow tube reactor adopts a Ni tube with an inner diameter of 20mm and a length of 800mm. The composite catalyst is packed into the plug-flow tube reactor. and placed in a heating furnace to heat up to 350°C for reaction. The reaction pressure is normal p...
Embodiment 2
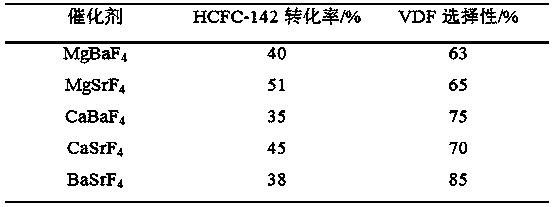
[0035] First, take 0.01 mol of each of the two alkaline earth metal nitrates and put them into a container, and add 100 mL of water. After mixing and stirring evenly, add 0.04mol solid NH 4 F formed a precipitate and was filtered after continued stirring for 2 h. The solid obtained after filtration was dried at 110° C. for 12 hours, and calcined at 500° C. for 4 hours to obtain a composite catalyst of alkaline earth metal fluoride.
[0036] Then, the obtained composite catalyst catalyzes the removal of HCl from 1,1-difluoro-1-chloroethane to prepare vinylidene fluoride. The reaction is carried out in a plug-flow tube reactor. The reaction tube of the plug-flow tube reactor adopts a Ni tube with an inner diameter of 20mm and a length of 800mm. The composite catalyst is packed into the plug-flow tube reactor. and placed in a heating furnace to heat up to 350°C for reaction. The reaction pressure is normal pressure.
[0037] Before the reaction, N 2 (airspeed is 1000h -1 ),...
Embodiment 3
[0041] First, take 0.01mol each of the two alkaline earth metal hydroxides and add them to the grinding, mix and stir evenly, then add 0.02mol (NH 4 ) 2 SiF 6 , and continue grinding for at least 0.5h, the obtained solid was dried at 110°C for 12h, and calcined at 500°C for 4h to obtain a composite catalyst of alkaline earth metal fluoride.
[0042] Then, the obtained composite catalyst catalyzes the removal of HCl from 1,1-difluoro-1-chloroethane to prepare vinylidene fluoride. The reaction is carried out in a plug-flow tube reactor. The reaction tube of the plug-flow tube reactor adopts a Ni tube with an inner diameter of 20mm and a length of 800mm. The composite catalyst is packed into the plug-flow tube reactor. and placed in a heating furnace to heat up to 350°C for reaction. The reaction pressure is normal pressure.
[0043] Before the reaction, N 2 (airspeed is 1000h -1 ), the temperature of the composite catalyst bed was raised from room temperature to 200°C at a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com