Preparation method of difluorophosphoric acid alkali metal salt
A technology of difluorophosphate alkali and hexafluorophosphate, which is applied in the direction of phosphorus halide/oxyhalide, etc., can solve the problems of difficult handling, high product cost, expensive, etc., and achieve the effect of ensuring safety and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
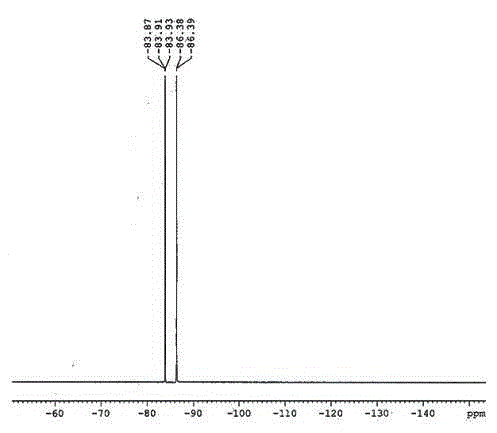
[0019] Weigh 11.06g of lithium hexafluorophosphate and 8.02g of lithium phosphate in an argon-protected glove box and add them to a hydrothermal reaction kettle with a polytetrafluoroethylene liner, mix them on a double-roll mixer for 0.5 hours, and then put the hydrothermal reaction The kettle was put into a forced air drying oven, set at 150°C and heated for 12 hours, and naturally cooled to room temperature after the reaction was completed. Take the material out of the glove box into a Teflon beaker, add 150g of acetone and stir for 1 hour, then remove the solids by filtration under reduced pressure, transfer the filtrate to a rotary evaporator for evaporation and crystallization, and gradually precipitate from the crystals as the solvent volatilizes . Subsequently, the evaporated and crystallized product was transferred to a vacuum drying oven for further drying treatment to obtain 11.42 g of the product, with a yield of about 85%. product 19 FNMR and ion chromatography ...
Embodiment 2
[0021] Weigh 55.46g of lithium hexafluorophosphate, 38.14g of lithium phosphate and 298g of ethylene glycol dimethyl ether in a glove box in an argon atmosphere, add them to a polytetrafluoroethylene flask, add a stirring magnet, and add a polytetrafluoro tube above the flask for air condensation Reflux, while the polytetrafluoro tube is externally connected to the drying tower emptying device. The reaction device was placed in an oil bath, and reacted for 10 hours at a temperature of 90° C. accompanied by magnetic stirring. After the reaction was completed, it was naturally cooled to room temperature. The solid was removed by filtration under reduced pressure, and the filtrate was transferred to a rotary evaporator to evaporate the solvent ethylene glycol dimethyl ether. With the evaporation of the solvent, the crystals were gradually precipitated, and finally the precipitated crystals were transferred to a vacuum drying oven to continue drying. The drying method was 60 The g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com